Общая характеристика
Бензодиазепины (БЗД) – часто назначаемые лекарственные средства как рецептурные, так и off-line [1].
С момента внедрения в клиническую практику (мепробомат, 1955) транквилизаторы, в частности БЗД, входят в число наиболее распространенных препаратов во всем мире. В какой-то мере транквилизаторы, БЗД и антидепрессанты, ингибиторы захвата серотонина являются наследниками опиума, марихуаны и морфина, широко доступных в XIX в., а в последующем и барбитуратов [2]. Часть из них применяется как снотворное, другие – как транквилизаторы [3].
В большей или меньшей степени БЗД свойственно противосудорожное действие, поэтому некоторые из них используют исключительно для борьбы с эпилепсией. Они также эффективны для лечения панических атак, вызванных приемом наркотиков-галлюциногенов. БЗД входят в широкую группу депрессантов центральной нервной системы, по анатомо-терапевтическо-химической классификации имеют код N05BA. Особое внимание привлекают БЗД как средства для купирования синдрома отмены алкоголя [4, 5].
Классификация
В настоящее время в лекарственном обороте находятся cвыше 55 производных бензодиазепина, главным образом в таблетированной форме или капсулах. Применяются также инъекционные формы (основной путь введения для мидазолама). Среди БЗД, представленных на мировом рынке, около 40 находятся под международным контролем Конвенции психотропных веществ. Ниже приводится несколько видов классификации БЗД по тому или иному признаку.
I. Химическое строение [6]:
1. Производные 1,4-бензодиазепина (хлордиазепоксид, диазепам, клоназепам, лоразепам, оксазепам).
2. Производные 1,5-бензодиазепина (клобазам, трифлубазам).
3. Трициклические производные, т.н. триазолбензодиазепины (алпразолам, адиназолам, эстазолам, лопразолам, триазолам).
4. Производные тиенодиазепина (бротизолам, клотиазепам).
5. Имидазолбензодизепины (мидазолам).
II. Длительность действия [7], не путать с периодом полувыведения (Т1/2): так, для клоназепама: Т1/2=19–60 часов, длительность действия (фармакологический эффект) 6–12 часов.
1. БЗД короткого действия – 1–12 часов (бротизолам, мидазолам, триазолам). Обладают некоторым остаточным эффектом, если принимаются перед сном, возобновление бессонницы может произойти после их отмены. Они могут вызывать симптомы отмены на следующий день в виде усиления тревожности при длительном использовании, имеют малый аддитивный потенциал.
2. БЗД средней продолжительности действия – 12–40 часов (алпразолам, бромдигидрохлорфенил бензодиазепин, эстазолам, флунитразепам, клоназепам, лорметазепам, лоразепам, нитразепам, темазепам). Они могут иметь некоторый остаточный эффект в первой половине дня, если применяются как снотворное. Возобновление бессонницы чаще возникает при прекращении приема БЗД средней продолжительности действия, чем длительного.
3. БЗД длительного действия – 40–250 часов (диазепам, гидазепам, медазепам, клоразепат, хлордиазепоксид, флуразепам). При их приеме есть риск накопления в пожилом возрасте и у лиц с тяжелыми нарушениями функции печени, но они вызывают менее выраженный синдром отмены.
III. БЗД в зависимости от пути метаболизма условно разделяются на две группы. Первая и самая большая группа включает БЗД, биотрансформация (I фаза метаболизма) которых катализируется изоформами фермента цитохрома P-450 (CYP) и они обладают значитель-ным потенциалом для взаимодействия с другими лекарственными средствами. Вторая группа метаболизируется через связывание с глюкуроновой кислотой (фермент UDP-глюкуроносульфо-трансфераза) (II фаза метаболизма) и в целом реже вызывает лекарственные взаимодействия. К таким препаратам относят, например, лоразепам, оксазепам и темазепам.
Фармакология и токсикология
Действие БЗД связано с воздействием на рецепторы ГАМК (γ-аминомасляной кислоты). Они выступают в роли позитивных аллостерических модуляторов: соединяются с рецептором ГАМК-А в области между α- и γ-субъединицами, в результате чего изменяется конформационная структура рецептора, увеличивается частота открытия каналов для хлорид-ионов, тем самым потенцируется эффект ГАМК на собственные рецепторы [8]. Чувствительность рецепторов к БЗД обусловлена наличием описанной выше γ-2 субъединицы в составе рецептора. Активация ГАМК-А рецепторов означает гиперполяризацию мембранного потенциала нейрона и его торможение. Большинство БЗД имеют равный аффинитет к рецепторам ГАМК, содержащим субъединицы подтипов α-1, α-2, α-3 или α-5. Рецепторы с α-4 и α-6 субъединицами практически не чувствительны к бензодиазепиновым транквилизаторам.
Высокая распространенность БЗД также приводит к злоупотреблению ими. Несмотря на то что передозировка данными веществами происходит значительно чаще по сравнению с любыми другими сильнодействующими веществами, БЗД считаются относительно безопасными благодаря способности человеческого организма достаточно быстро адаптироваться к повышенному содержанию БЗД в крови. При этом следует учитывать, что прием БЗД становится небезобидным в сочетании с алкоголем или другими успокоительными средствами, антидепрессантами, нейролептиками и морфиноподобными веществами, что приводит к возникновению синергетического эффекта. Употребление БЗД в сочетании с алкоголем особенно опасно, т.к. данная комбинация может приводить к летаргии, что увеличивает вероятность возникновения дорожно-транспортных происшествий и инцидентов, связанных с домашним насилием [9].
При долговременном использовании препараты могут вызывать привыкание и физическую зависимость. Наиболее частые побочные эффекты БЗД связаны с их седативным и миорелаксирующим действиями: сонливость, головокружение, снижение внимания и концентрации. Нарушение координации может приводить к падениям и травмам, особенно у пожилых людей. БЗД могут обострять депрессивную симптоматику и увеличивать риск суицидального поведения [10].
Кинетика и биотрансформация
БЗД в основном характеризуются хорошей биодоступностью. Максимальная концентрация в плазме достигается через 1–4 часа после приема внутрь. Препараты интенсивно (80–97%) связываются с белками плазмы крови. Подробные сведения по фармакокинетике БЗД приведены в обстоятельном руководстве [11]. Параметры фармакокинетики некоторых распространенных в клинической практике БЗД приведены в табл. 1. Значения Т1/2 и клиренса характеризуются значительными разбросами и отличаются от препарата к препарату.
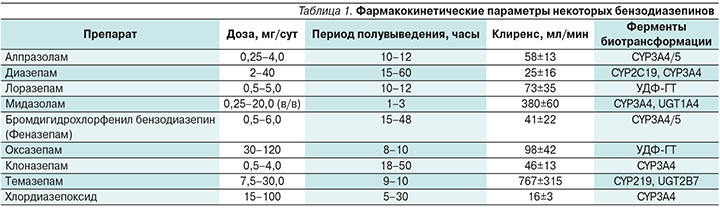
Метаболизм БЗД включает две фазы: фаза I подразумевает введение полярной группы в исходную молекулу путем окисления, восстановления или гидролиза, фаза II – конъюгацию лекарственного средства с полярной молекулой (ацетилирование, глюкуронизация) [12]. Большинство БЗД (кроме лоразепама и оксазепама) метаболизируются в I фазу изоферментами системы цитохрома P-450, и метаболиты в той или иной степени обладают фармакологической активностью. Лоразепам и оксазепам подвергаются только глюкуронизации (фермент UDP-glucuronosyltransferase) и затем выводятся почками, поэтому активность изоферментов цитохрома P-450 не влияет на их фармакокинетику. Следует отметить, что в результате биотрансформации происходит превращение одних лекарственных веществ в другие. Так, диазепам является «пролекарством» в широком смысле этого слова для нордиазепама, оксазепама, и темазепама. С другой стороны, оксазепам является активным метаболитом таких БЗД, как диазепам, кетазолам, темазепам, хлордиазепоксид, демоксазепам [13].
Фармакогенетика
БЗД метаболизируются ферментами CYP2D6, CYP3A4/5 и CYP2C19. Персонализированный подбор дозы обязательно должен разрабатываться с учетом генотипов CYP2D6, CYP3A4/5 и CYP2C19. Не менее важным является учет и фармакодинамических полиморфизмов, кодирующих «мишени» действия транквилизаторов: гена GABRA.
Количественное определение
Вышеуказанные свойства и области применения БЗД в современной сфере жизнедеятельности человека обусловливают требования к количественному анализу этих веществ. Одним из основных приложений инструментальных методов являются наркология и судебная медицина – лекарственная токсикология, связанная с определением пороговой дозы того или иного БЗД, используемого в качества наркотического средства. В данной публикации основное внимание уделяется клинической фармакокинетике и терапевтическому лекарственному мониторингу БЗД. В связи с этим в качестве основного матрикса используются цельная кровь [14], сыворотка [15] и плазма [16].
Главными факторами при проведении анализа является наличие молекул целевых БЗД или их метаболитов. Независимо от выбора матрикса необходимо учитывать, что уровень концентрации БЗД зависит от полученной дозы, режима дозирования, качества препарата, а также индивидуальных характеристик пациента – скорости метаболизма, массы тела, комедикации и т.д. Принято различать два вида анализа на содержание БЗД: 1) скрининговые (качественные и полуколичественные методы); 2) дозовые (количественные методы). При этом токсикологический скрининг обычно включает несколько скрининговых и дозовых методов.
Пробоподготовка
Депротеинизация матрикса достигается преципитацией белков, заключающейся в смешивании одного объема плазмы/сыворотки крови с тремя объемами: органического растворителя – ацетона, метанола, ацетонитрила [17] с последующим перемешиванием и центрифугированием, что приводит к осаждению 99% белков. Добавление октилсульфата или додецилсульфата натрия, разрушающих структуру белков, является альтернативным способом отделения БЗД [18]. Также возможно использование методов ультрамикрофильтрации и равновесного диализа для отделения белков от образцов крови [19], однако ультрамикрофильтрат и диализат содержат лишь свободную фракцию БЗД.
Жидкостная экстракция. Наиболее часто используемый подход, обусловленный выраженной гидрофобностью БЗД. Для проведения жидкостной экстракции предложена масса растворителей и их смесей в щелочной и намного реже в слабощелочной или кислой среде. Наиболее употребительными являются гексан, метил-трет-бутиловый эфир, н-бутилхлорид, дихлорметан, гексан ‒ дихлорметан (70:30 по объему) и этилацетат [19, 20]. Для перехода вещества (особенно метаболитов) в неионизированную форму добавляют растворы гидроксида натрия, карбоната натрия или боратный буфер.
Твердофазная экстракция. В принципе твердофазная экстракция основана на тех же принципах, что и жидкостная хроматография. Главные различия: короткие одноразовые колонки (патроны, картриджи) и большой размер зерна сорбента. Данный подход в отличие от жидкостной экстракции позволяет выделять гидрофильные компоненты, характерные для метаболизма БЗД. Патроны для твердофазной экстракции: Oasis HLB (Waters), Oasis MCX (Waters), Strata® Screen-C (Phenomenex) SOLA, (Thermo). Следует отметить, что при этом появляется возможность автоматизации экстракции, реализуемой в виде различных роботизированных платформ [21].
Жидкостная экстракция в нанесенном слое (англ. SLE – Solid supported liquid-liquid extraction). Процедура основана на извлечении интересующего компонента из водного образца в слой жидкости, распределенной на твердом высокополярном носителе с последующим элюированием системой неполярных растворителей (например, этилацетат, метил-трет-бутиловый эфир), не смешивающихся с этим слоем [22, 23].
Стоит также отметить, что в некоторых случаях количественного определения БЗД в биологических образцах (чаще в моче) вообще обходятся без экстракционных процедур [24].
Инструментальные методы
Применяются разнообразные методы: инфракрасная спектроскопия, Рамановская спектроскопия [25], ядерный магнитный резонанс, капиллярный электрофорез. Подробное описание этих методов выходит за рамки данной публикации. Тем не менее следует отметить позитронную эмиссионную томографию (ПЭТ) – быстро развивающийся метод визуализации биораспределения [26] и количественного определения лекарственных веществ. Для реализации возможностей метода, прежде всего необходимы радиотрейсеры, биологически активные соединения, содержащие в своем составе короткоживущие ПЭТ-радиоизотопы [27].
Хроматографические методы
Газовая хроматография (ГХ). Наличие галогенов и азота в химической структуре БЗД обусловливает применение ГХ для многих аналитов среди этого класса соединений. Для разделения БЗД на капиллярных колонках используют умеренные неполярные фазы: диметилсиликон (OV-1, OV-17) или 50% фенилсиликон. В качестве детектора рекомендуются электронно-захватный детектор (ECD) и термоионный (NPD) детектор, (иначе: азот-фосфорно-селективный детектор). Однако для многих веществ, особенно метаболитов, наличие гидрокси- и аминогрупп снижает эффективность ГХ. В этих случаях используется дериватизация образцов: как правило, ацильные и алкильные производные БЗД [28].
Высокоэффективная жидкостная хроматография (ВЭЖХ). Для анализа БЗД в биологических пробах методом ВЭЖХ используются обращено-фазные колонки с сорбентами С8 или С18. Подвижные фазы – кислые, смесь фосфатного буфера и ацетонитрила и/или метанола, применяемые в изократическом или градиентном режиме элюирования. Очень важен контроль рН подвижной фазы (стабильность рН), т.к. небольшая разница значительно влияет на результаты хроматографического разделения. Для сложных смесей БЗД необходим градиентный режим или комбинация изократических потоков подвижной фазы. Несмотря на все более повсеместное использование масс-спектрометрического детектора (масс-спектрометр – МС) в методиках количественного определения БЗД, спектрофотометрия в ультрафиолет (УФ)-видимом диапазоне остается достаточно популярной и также часто применяется. Этому способствуют широкие терапевтические интервалы дозирования рассматриваемой группы препаратов. При УФ-детекции БЗД выделяют 3 полосы интенсивного поглощения: 200–215 нм, 220–240 и 290–330 нм [29] (табл. 2). Для лучшей идентификации смеси БЗД требуется диодно-матричное детектирование [30]. В ряде случаев используется флуоресцентный детектор. Находит свое применение и электрохимический детектор [31].
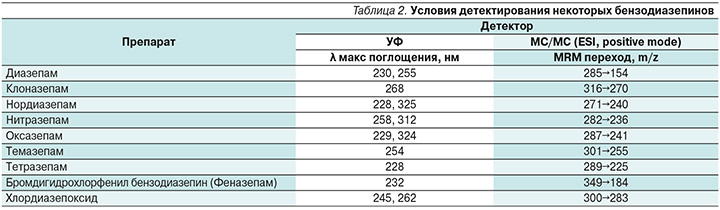
Масс-спектрометрическое (МС) детектирование. Масс-селективные детекторы, такие как квадрупольные, времяпролетные МС или по типу ионной ловушки, часто применяются в комбинации с капиллярной ГХ или газо-жидкостной хроматографией (ГЖХ) [32, 33]. Современные колонки (например, Agilent DB-5MS) с внутренним диаметром 0,03–0,53 мм и длиной 5–50 м позволяют проводить одновременное определение нескольких нативных БЗД и их активных метаболитов [34]. При дериватизации силильные производные БЗД имеют преимущества в МС в связи с тем, что дают стабильные ионы с высокими молекулярными массами. ГЖХ-МС считается «золотым» стандартом для судебно-медицинской идентификации веществ, однако в фармакологических экспериментах ее возможности представляются ограниченными. По той же причине, что и ВЭЖХ, все больше вытесняет ГХ из фармацевтического биоанализа, в гибридных подходах сочетание жидкостной хроматографии с МС становится преобладающим методом анализа биологических образцов. Дело в том, что данный подход позволяет анализировать нелетучие и термонестабильные вещества без трудоемкой дериватизации. При этом определяемые компоненты сначала разделяют на хроматографической колонке по времени удерживания, а затем на масс-анализаторе по массам исходного иона и специфического фрагмента. Аналиты переводят в ионы путем электрораспыления (ESI – electrospray ionization) – ионизация электроспреем или химической ионизации при атмосферном давлении (APCI – atmospheric pressure chemical ionization). Наиболее распространенными подвижными фазами для ВЭЖХ-МС являются смеси водных растворов аммониевых солей, муравьиной или уксусной кислоты с органическими растворителями (ацетонитрил, метанол). В то же время категорически противопоказано использование фосфатных и других неорганических буферов. Уже достаточное время этот метод, особенно в тандемном варианте (ВЭЖХ-МС-МС), в котором возможно осуществлять регистрацию масс-спектров в МRM-режиме (Multiple Reaction Monitoring – режим мониторинга множественных реакций), является наиболее предпочтительным в клинико-фармакологических исследованиях [35] (величины протонированных материнских ионов и их специфических фрагментов для регистрации в режиме положительных ионов при ESI некоторых БЗД приведены в табл. 2).
Как и в случае с ГХ-МС предпочтительно определять линейку минимум 10–20 аналитов [14, 17, 21]. Следует отметить, что для определения метаболитов БЗД целесообразно использовать хроматографические колонки с гидрофобным взаимодействием (фаза HILIC) [36].
Иммуноферментный анализ (ИФА)
ИФА является одним из основных методов рационального выбора при скрининге БЗД в токсикологической и наркологической практике, когда необходимо проанализировать несколько образцов в кратчайшее время для получения негативного или позитивного ответа. Различают несколько разновидностей ИФА (EIA, enzyme immunoassay): метод ИФА с умножением ферментов (EMIT – enzyme multiplied immunoassay technique), ИФА с использованием связанного с ферментом сорбента (ELISA – enzyme linked immunoadsorbent assay), гомогенный ИФА (HEIA – homogeneous enzyme immunoassay), кинетический ИФА микрочастиц в растворителе (KIMS – kinetic interaction of microparticles in solution) и ИФА донора клонированных ферментов (CEDIA – cloned enzyme donor immunoassay) [6]. Благодаря скорости и простоте использования ИФА обычно используются в качестве метода скрининга перед подтверждением с использованием ЖХ-МС. ИФА широко используются в судебной токсикологии благодаря их простоте использования, быстроте, гибкости и возможности получения полуколичественных результатов. Многие из этих анализов достаточно гибки, чтобы реагировать на различные структуры БЗД. Однако в некоторых случаях большое разнообразие химических структур БЗД в одной анализируемой пробе приводит к ложноположительным или ложноотрицательным результатам из-за переменной иммунореактивности антител, используемых для ИФА. Например, в анализе EMIT® II Plus на бензодиазепин в исследовании [37] используются поликлональные овечьи антитела, нацеленные на диазепам в качестве сайта связывания. Лекарственное средство, присутствующее в биологическом образце, конкурирует с меченым диазепамом, который добавляется в качестве субстрата при анализе. Некоторые БЗД, такие как бромазепам, лоразепам и особенно метаболиты БЗД II фазы – конъюгаты с глюкуроновой кислотой, проявляют низкую перекрестную реактивность, т.е. они обладают меньшим сродством к поликлональному антителу, чем меченый диазепам, что увеличивает вероятность получения ложноотрицательных результатов [38]. Ферментативный гидролиз биологического матрикса (мочи) с использованием β-глюкуронидазы для высвобождения конъюгированных БЗД улучшает обнаруживаемость последних в ИФА [39]. Более того, даже если ИФА способны различать положительные и отрицательные образцы, полуколичественная информация приблизительна, поскольку она отражает совокупные концентрации лекарств и их метаболитов, которые вступают в перекрестную реакцию с анализом [37].
Заключение
ИФА и ЖХ-МС-МС имеют свои достоинства, главными из которых являются возможность автоматизации в процессе ИФА и быстрый переход с одного вида анализа на другой при ЖХ-МС-МС. К числу недостатков следует отнести перекрестную контаминацию в случае ИФА, ионную супрессию и матричный эффект для ЖХ-МС-МС [40]. В исторической перспективе проведение терапевтического лекарственного мониторинга ограничивалось измерением концентрации одного целевого вещества. Применение ЖХ-МС-МС позволяет одновременно определять несколько БЗД и их активных метаболитов в пробе [21, 41]. Кроме того, появляется возможность в том же образце параллельно измерять содержание соответствующих биомаркеров (ФК-ФД-мониторинг) [42] или сопутствующих в терапии других лекарственных средств [43].
Финансирование. Выполняемая по госзаданию плановая НИР «Ком-плексный мониторинг параметров фармакокинетики и фармакодинамики при терапии психических заболеваний».
Дополнительная информация
Публикация статьи осуществлялась в рамках диссертационной работы Кузьмина Ивана Игоревича на соискание ученой степени канд. биол. наук по специальности 14.03.06 – фармакология, клиническая фармакология: «Терапевтический лекарственный мониторинг феназепама и венлафаксина».



