Введение
Синдром поликистозных яичников (СПКЯ) является наиболее распространенным эндокринным заболеванием, которое выявляется у 5–25% женщин репродуктивного возраста [1, 2], при этом до 70% случаев остаются недиагностированными в результате значительной клинической гетерогенности [3]. Клинические проявления СПКЯ могут включать репродуктивные нарушения (нарушения менструального цикла, бесплодие, осложнения беременности), метаболические (инсулинорезистентность, метаболический синдром, сахарный диабет 2 типа, апноэ во сне, сердечно-сосудистые заболевания), психологические (депрессия, тревожность, нарушение пищевого поведения, сниженная самооценка) и проявления гиперандрогении (гирсутизм, акне, алопеция) [4].
Согласно международному консенсусу, диагноз СПКЯ устанавливается на основании Роттердамских критериев [5], среди которых гипер-андрогения клиническая и/или биохимическая, овуляторная дисфункция и поликистозные яичники по данным ультразвукового исследования (УЗИ). Для подтверждения диагноза СПКЯ необходимо наличие двух из них при исключении других причин нарушений менструального цикла и гиперандрогении, таких как заболевания щитовидной железы, гиперпролактинемия, неклассическая форма врожденной гиперплазии надпочечников и более редких – гипогонадотропный гипогонадизм, болезнь Кушинга и андрогенпродуцирующие опухоли.
Гиперандрогения
Основные этапы яичникового стероидогенеза происходят в тека-клетках стромы, где под влиянием пульсаторной секреции лютеинизирующего гормона (ЛГ) происходит синтез андрогенов – андростендиона и тестостерона (рис. 1). При созревании антральных фолликулов под контролем фолликулостимулирующего гормона (ФСГ) андрогены диффундируют через мембрану из теки в гранулезный слой, где с помощью фермента ароматазы преобразуются в эстрогены. При СПКЯ секреция ФСГ значительно уступает ЛГ. Избыток или чрезмерная пульсация ЛГ активирует рецепторы в клетках гранулезы, вызывает преждевременную остановку роста антральных фолликулов и их накопление в яичнике. Одновременно ЛГ вызывает избыточную пролиферацию тека-клеток яичников и продукцию ими андрогенов, при этом подавляя активность ароматазы, конвертирующей андрогены в эстрогены, тем самым усиливая гиперандрогенизм. Таким образом, нарушение стероидогенеза в яичниках приводит к увеличению выработки андрогенов и клиническим проявлениям гиперандрогении – гирсутизму, акне и/или алопеции [6]. По механизму отрицательной обратной связи гиперандрогения ведет к аномальной реакции ЛГ на триггерное воздействие гонадотропин-рилизинг гормона (ГнРГ) и нарушению центральных механизмов регуляции гипоталамо-гипофизарно-яичниковой оси [7]. В большинстве случаев аномальная реакция формируется в период внутриутробного развития и ассоциирована в первую очередь с повышенной выработкой антимюллерова гормона (АМГ) у беременных женщин с СПКЯ. Кроме непосредственного стимулирующего влияния на пульсационный выброс ЛГ АМГ ингибирует активность ароматазы в плаценте, тем самым повышая биодоступность андрогенов для плода. Таким образом, под влиянием гиперандрогенного статуса у матери происходит андроген-ассоциируемое перепрограммирование нейронального ответа на ГнРГ и рождение ребенка с нарушенным чрезмерным синтезом ЛГ и высоким риском развития СПКЯ [8].
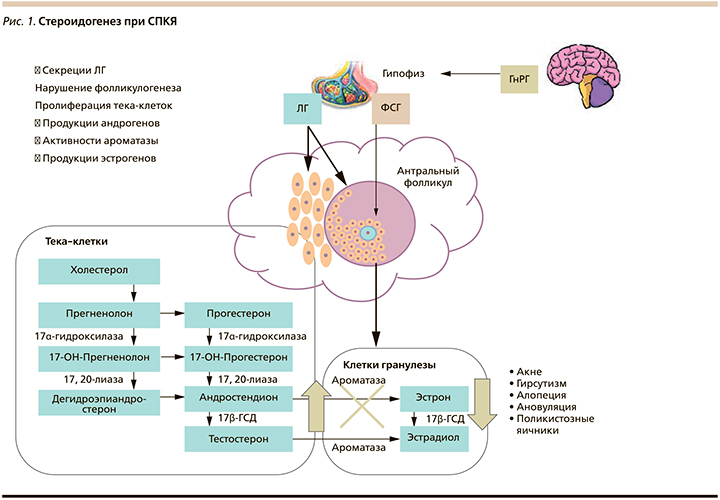
Клиническая гиперандрогения. Кли-ническими признаками гиперандрогении являются гирсутизм, акне и алопеция. Повышенное оволосение встречается у 65–75% женщин с СПКЯ [9]. Оно определяется при появлении темных волос в местах, характерных для мужского телосложения (верхняя губа, подбородок, щеки, грудь, спина, область живота, руки, ноги), степень которого оценивается по шкале Ферримана Галлвея. В то же время у 3–35% женщин [10] может отмечаться потеря волос (алопеция) в области волосистой части головы. Почти половина пациенток с СПКЯ жалуются на акне [11]
Биохимическая гиперандрогения
Основным андрогеном яичников является тестостерон, который, поступая в кровь, связывается с глобулином, связывающим половые гормоны (ГСПГ), альбумином и другими белками, в небольшом количестве оставаясь в свободной форме, и достигает органов-мишеней [12]. К биохимическим маркерам гиперандрогении яичникового генеза относят уровень общего тестостерона, свободного тестостерона или индекс свободных андрогенов в крови. Сывороточный уровень андростендиона и дегидроэпиандростерона сульфата также может быть использован у женщин с клиническими проявлениями гиперандрогении, но с нормальными показателями тестостерона. При этом следует проводить дифференциальную диагностику с надпочечниковой гиперандрогенией [13].
Определенную помощь в диагностике СПКЯ может оказывать оценка уровня гонадотропных гормонов гипофиза, регулирующих стероидогенез в яичниках. У многих женщин с СПКЯ секреция ЛГ в первую фазу менструального цикла превышает секрецию ФСГ, что приводит к повышению соотношения ЛГ/ФСГ>2 [14]. Дополнительным маркером заболевания также может служить повышенный уровень АМГ [15], секретируемого преантральными фолликулами, в большом количестве представленными в яичниках при СПКЯ.
У женщин, принимающих гормональные контрацептивы, под влиянием которых деятельность гипоталамо-гипофизарной оси подавляется, уровень андрогенов и гонадотропных гормонов оценивают не ранее чем через 3 месяца после их отмены.
Овуляторная дисфункция
Овуляторная дисфункция (отсутствие овуляции) определяется у 70–80% женщин с СПКЯ [16] и обычно характеризуется нарушениями менструального цикла, такими как аменорея или олигоменорея (<8 менструаций в год), нередко в сочетании с меноррагией. СПКЯ выявляется у 91% женщин с нерегулярным менструальным циклом [17], повышая риск бесплодия в 15 раз [18]. Ановуляция может присутствовать и при регулярном менструальном цикле [9]. Для ее подтверждения проводится УЗИ яичников или измеряется сывороточный уровень прогестерона во вторую фазу цикла [16]. Следует также учитывать, что у девочек-подростков нерегулярный менструальный цикл и/или овуляторная дисфункция являются характерной особенностью и часто не связаны с диагнозом СПКЯ.
Поликистозные яичники по данным УЗИ
Поликистозная морфология яичников определяется с помощью УЗИ при наличии ≥20 фолликулов в каждом яичнике. Преимущественно они локализуются по периферии в виде «ожерелья», окружающего гиперплазированную строму. Также к критериям СПКЯ относится увеличение объема яичников ≥10 мл3 в отсутствие в них доминантных фолликулов, кист или желтого тела [19]. Морфологические изменения в яичниках у большинства женщин с СПКЯ не соответствуют названию, т.к. не являются кистами, а представлены большим числом антральных и преантральных фолликулов [20].
Не рекомендуется использовать ультразвуковые данные в диагностике СПКЯ в течение 8 лет после менархе в связи с высокой частотой мультифолликулярных яичников в этом периоде.
Критерии диагностики СПКЯ
Таким образом, для диагностики СПКЯ, согласно обновленным рекомендациям, необходимо наличие двух из трех представленных ниже критериев [4, 21]:
1. Овуляторная дисфункция (менструальный цикл <21 или >35 дней, или олиго-ановуляция по данным УЗИ).
2. Гиперандрогения клиническая (акне/гирсутизм) и/или биохимическая (повышенный сывороточный уровень свободного тестостерона, индекса свободных андрогенов или общего тестостерона).
3. Поликистозные яичники по данным УЗИ (≥20 фолликулов в каждом яичнике и/или объем яичника ≥10 мл3).
Инсулинорезистентность
В последние годы СПКЯ стал рассматриваться не только как заболевание, связанное с репродукцией, но и все чаще в контексте метаболических нарушений, в основе которых лежит инсулинорезистентность (ИР). ИР имеют от 50 до 80% женщин с СПКЯ [22, 23], избыточную массу тела – 61% [24], сахарный диабет 2 типа и метаболический синдром до 40 лет – более половины [25].
При ИР изменяется стероидогенез в коже и жировой ткани. За счет активации ароматазы, конвертирующей андрогены в эстрогены, периферический синтез эстрогенов повышается и по механизму отрицательной обратной связи снижается выброс ФСГ и его влияние на рост фолликулов в яичниках [2, 26] (рис. 2). При этом яичники в отличие от других органов и тканей всегда сохраняют нормальную чувствительность к инсулину и поэтому при наличии системной ИР подвергаются воздействию компенсаторной гиперинсулинемии [27]. Высокий уровень инсулина снижает чувствительность гранулезных клеток и ооцитов к действию ФСГ, а через свои центральные рецепторы повышает пульсацию ГнРГ и ЛГ, тем самым дополнительно ингибируя фолликулогенез и созревание ооцитов [2]. Чрезмерная активация ЛГ и непосредственная стимуляция инсулином тека-клеток яичников вызывает их пролиферацию и синтез андрогенов, при этом в отличие от кожи и жировой ткани, находящихся в условиях ИР, активность ароматазы, конвертирующей андрогены в эстрогены, подавляется. Также гиперинсулинемия усиливает продукцию андрогенов надпочечниками и ингибирует образование ГСПГ в печени, увеличивая фракцию свободных андрогенов в крови, приводя к клиническим проявлениям гиперандрогении и нарушениям менструального цикла [26, 28].
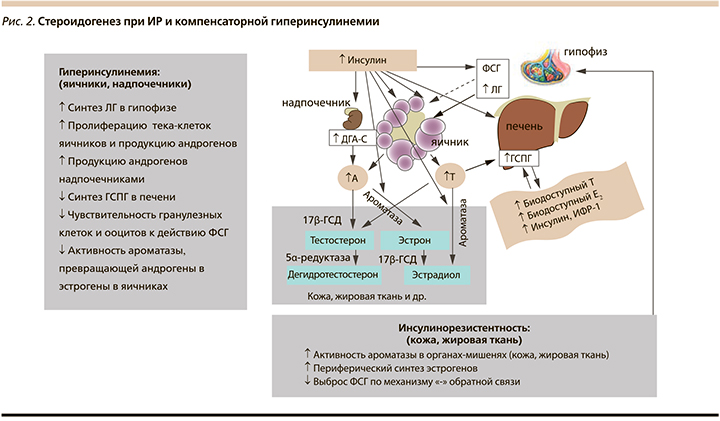
Таким образом, ИР и компенсаторная гиперинсулинемия становятся триггером основных патогенетических изменений при СПКЯ.
Помимо прямого участия в стероидогенезе инсулин косвенно также оказывает на него влияние через изменение соотношения стереоизомеров инозитола в ткани яичника – мио-инозитола (МИ) и Д-хироинозитола (ДХИ). МИ и ДХИ являются вторичными мессенджерами инсулина в клетках, где МИ отвечает за внутриклеточный транспорт глюкозы и параллельно конвертируется в ДХИ, необходимый для включения ее в цикл Кребса или депонирования. Инсулин стимулирует активность фермента эпимеразы, ответственной за превращение МИ в ДХИ, регулируя тканеспецифическое внутриклеточное соотношение МИ и ДХИ, которое отвечает за модуляцию различных метаболических процессов [29, 30].
Физиологическое соотношение МИ и ДХИ в сыворотке крови составляет 40:1 [31], тогда как в яичниках 100:1 [29], что обусловлено активным участием МИ в реализации сигналов ФСГ. При гиперинсулинемии эпимеризация МИ в ДХИ усиливается, что вызывает дефицит МИ с нарушением фолликулогенеза, ановуляцией и снижением качества яйцеклеток [32]. Увеличение ДХИ в свою очередь вследствие подавляющего эффекта на активность ароматазы нарушает конвертацию андрогенов в эстрогены, вызывая гиперандрогенное состояние у женщин с ИР [33].
Таким образом, гиперинсулинемия, развивающаяся у женщин с ИР, оказывает непосредственное влияние на гиперпродукцию андрогенов яичниками [34]. Более того, в настоящее время широко дискутируется тот факт, что гиперандрогения и ассоциированные с ней репродуктивные проблемы у женщин с СПКЯ являются следствием метаболических нарушений, а не гинекологического статуса и потому часто называют «метаболической гиперандрогенией», или «метаболическим репродуктивным синдромом». В то же время следует отметить и взаимное влияние повышенного уровня андрогенов на развитие ИР. Гиперандрогения способствует накоплению и перераспределению жировой ткани у женщин, вызывая повреждение сигнальных путей инсулина на клеточном уровне [35], и является независимым фактором риска нарушения метаболизма глюкозы и развития ИР [36].
В исследовании С. Alviggi et al. [37] продемонстрированы различия морфологической структуры яичников у женщин с СПКЯ при наличии и отсутствии ИР. «Классические» поликистозные яичники с «ожерельем» из антральных фолликулов размером 5–9 мм и плотной стромой в соотношении с общей площадью яичников >0,34 наблюдались у 78,1% женщин с гиперандрогенией без ИР. У 87,8% гиперандрогенных женщин с ИР яичники были большего объема (р=0,024) с множеством хаотично расположенных мелких фолликулов размером 2–4 мм и соотношением площади стромы к яичникам <0,34. Также у них отмечалось значительное увеличение индекса массы тела (ИМТ; р=0,0001) при более низком уровне ГСПГ (р=0,0001) и ЛГ (р=0,002). На основании полученных данных авторы указывали на необходимость более детальной оценки морфологической структуры яичников с разделением СПКЯ на гиперандрогенный и гиперинсулинемический фенотипы, что может помочь более дифференцированно определять тактику ведения таких пациенток.
Исключительно важно результаты УЗИ яичников оценивать в сочетании с андрогенным статусом и метаболическими параметрами. В частности, у некоторых женщин, имеющих поликистозные яичники и овуляторную дисфункцию, уровень андрогенов, инсулина и глюкозы не отличается от таковых здоровых женщин [38]. Предполагается, что повреждение структуры и функции яичников в данном случае не связано с гиперандрогенией или ИР, а обусловлено локальной гиперпродукцией инсулиноподобного фактора роста-1 (ИФР-1), вызывающего преждевременную остановку роста доминантного фолликула и накопление в яичниках фолликулярных кист различного размера, секретирующих массу эстрогена [39]. Снижение выработки прогестерона при ановуляции в свою очередь вызывает недостаток экспрессии ИФР-связывающего белка-1 в эндометрии, блокирующего пролиферирующее влияние эстрогенов, что повышает риск развития гиперплазии и рака эндометрия [40].
Таким образом, для правильной диагностики и определения тактики ведения пациенток с СПКЯ требуются дополнительные диагностические маркеры, не ограничивающиеся только предложенными международным консенсусом Роттердамскими критериями [41].
Фенотипы СПКЯ
С учетом разнообразия клинической картины СПКЯ выделяют четыре фенотипа заболевания [4], среди которых только фенотип А характеризуется наличием всех трех диагностических критериев, а остальные устанавливаются при сочетании двух из них:
- Фенотип А («классический»): гиперандрогения+овуляторная дис-функция+поликистозные яичники.
- Фенотип В («гиперандрогенный ановуляторный»): гиперандрогения+овуляторная дисфункция.
- Фенотип С («овуляторный»): гиперандрогения+поликистозные яичники.
- Фенотип D («нормоандрогенный»): овуляторная дисфункция+поликистозные яичники.
К сожалению, разделение на фенотипы не привело к дифференциации терапевтических подходов, основная цель которых направлена на регуляцию менструального цикла, восстановление репродуктивного потенциала и избавление от косметологических проблем, обусловленных гиперандрогенией, без учета патогенетических различий. В основном помимо коррекции питания и образа жизни рекомендовались половые стероиды (циклические гестагены, гормональные контрацептивы), средства, стимулирующие овуляцию, вспомогательные репродуктивные технологии, а также различные методы лечения кожных проявлений гиперандрогении. При этом не учитывалось, например, что при фенотипе С репродуктивная функция не нарушена, а при фенотипе D отсутствуют любые признаки гиперандрогении. Также не были выделены группы женщин, наиболее приверженных терапии инсулинсенситайзерами, такими как метформин и инозитол.
В последние годы все больше экспертов признают необходимость пересмотра диагностических критериев и терапевтических походов при СПКЯ. Так, С. Alviggi et al. [37] предлагают дополнительно определять метаболические параметры женщин с гиперандрогенией, такие как ИМТ и индекс ИР, в соответствии с которыми подразделять на гиперандрогенный или гиперинсулинемический фенотип СПКЯ, проводить соответствующую патогенетически обоснованную терапию.
Другая группа ученых во главе с V. Unfer [42] разработала новую классификацию СПКЯ. Они предположили, что только фенотип D, имеющий особый патогенез повреждения структуры и функции яичников, не связанный с гиперандрогенией и/или ИР, является классическим заболеванием яичников. Более того, только в этой группе морфологические изменения в яичниках являются преимущественно кистами, а не пре- и антральными фолликулами, характерными для других ановуляторных фенотипов СПКЯ, и ассоциированы не только с бесплодием, но и с повышенным риском гиперплазии эндометрия вследствие локального дисбаланса экспрессии ИФР-1 и ИФР-связывающего белка-1 [39, 40]. Остальные фенотипы (А, В и С) в новой классификации были объединены в общую группу «метаболическая гиперандрогения», где основным этиопатогенетическим фактором выступает ИР. В этой группе женщин гиперандрогения и гинекологические проблемы рассматриваются не как причина, а скорее как следствие эндокринных и метаболических нарушений и ассоциированы с высоким риском развития сахарного диабета 2 типа, ожирения, метаболического синдрома и сердечно-сосудистых заболеваний.
Стратегии лечения
Таким образом, становится очевидным, что тактика ведения пациенток с СПКЯ не может быть универсальной и должна определяться как поставленными целями, направленными на нормализацию менструального цикла, преодоление бесплодия и/или лечение акне с гирсутизмом, так и различными этиопатогенетическими факторами развития заболевания, в т.ч. и ассоциированными с ними рисками. Перед назначением лечения необходимо не только установить диагноз СПКЯ, но и определить клинический фенотип заболевания с детальным анализом морфологической структуры яичников и дополнительным исследованием метаболических параметров, таких как ИМТ и индекс ИР в крови.
С учетом патогенетической роли ИР и компенсаторной гиперинсулинемии в развитии репродуктивных нарушений при СПКЯ у женщин с «метаболической гиперандрогенией» более широкое применение инсулинсенситайзеров, таких как метформин и инозитол, в дополнение к общим рекомендациям по коррекции пищевого поведения и физической активности представляется наилучшей терапевтической стратегией. Экспериментальные и клинические исследования показали, что МИ, один или вместе с ДХИ в соотношении 40:1, соответствующем физиологическому содержанию в плазме крови, является наиболее перспективным лечением метаболических, гормональных и репродуктивных нарушений у женщин с ИР и СПКЯ [43–45]. Соотношение инозитолов 40:1 содержится в препарате Актиферт-гино, представляющем собой растворимые таблетки, содержащие 1127,5 мг инозитола в виде 1100 мг МИ и 27,5 мг ДХИ в сочетании с 400 мкг фолиевой кислоты, назначаются по 1–2 в сутки. Шипучие растворимые таблетки имеют более высокую биодоступность и обладают лучшей абсорбцией в кишечнике по сравнению с порошковыми формами.
Не следует проводить монотерпию или увеличивать дозу ДХИ женщинам с СПКЯ, что ассоциировано с его негативным влиянием на активность ароматазы и еще большим увеличением продукции андрогенов яичниками [46]. Также не следует ожидать терапевтического эффекта от применения инозитола пациенткам без ИР и гиперандрогении [42].
Заключение
Несмотря на многолетние усилия ученых объединить и систематизировать клинические проявления СПКЯ, большинство случаев заболевания остаются недиагностированными.
В соответствии с клинической гетерогенностью выделяют четыре фенотипа заболевания, среди которых только фенотип А характеризуется наличием всех трех диагностических критериев (овуляторная дисфункция, гиперандрогения и поликистозные яичники). Фенотипы В, С и D устанавливаются по двум из них, но нередко игнорируются в клинической практике и потому пациентки не получают соответствующего лечения. Также разделение на фенотипы не привело к дифференциации терапевтических подходов, что отразилось на их недостаточной эффективности. На сегодняшний день назрела необходимость пересмотра диагностических критериев и лечебной тактики при СПКЯ с учетом этио-патогенетических факторов различных фенотипов, триггерной роли ИР и компенсаторной гиперинсулинемии в развитии многих случаев заболевания и более широким, но дифференцированным применением инсулинсенситайзеров.



