Эпидемиология
Анемия является одним из широко распространенных и потенциально опасных состояний у онкологических пациентов, негативно отражаясь на качестве и продолжительности их жизни. На момент постановки диагноза анемия той или иной степени тяжести диагностируется почти у каждого третьего пациента, нередко являясь одним из первых и основных клинических симптомов заболевания [1]. По другим данным, ее распространенность может достигать 90% и зависит главным образом от локализации первичной опухоли, стадии заболевания и метода противоопу-
холевого лечения [1, 2]. Пониженный уровень гемоглобина (Hb) чаще всего выявляется у пациентов с онкогематологическими заболеваниями, такими как лимфома и множественная миелома, а также с сóлидными новообразованиями, затрагивающими органы женской репродуктивной системы, желудочно-кишечного тракта (ЖКТ) и легкие [1]. Риски повышаются на поздних стадиях заболевания, а также при применении цитостатической терапии и/или лучевой терапии [1, 3].
Патогенез развития анемии при злокачественных новообразованиях
К потенциальным механизмам развития анемии у онкологического, как и любого другого пациента, относятся [4]:
- • острая или хроническая кровопотеря (постгеморрагические анемии);
- • нарушение эритропоэза (гипопролиферативные анемии);
- • ускоренный гемолиз (гемолитические анемии).
Вышеописанные механизмы могут быть вызваны различными причинами, однако зачастую анемия у онкологического пациента имеет смешанный генез (см. рисунок) [2, 5].

Анемия, связанная с наличием опухоли
При опухолях, локализующихся в полых органах, или при распадающемся процессе, часто происходит явное или скрытое (оккультное) кровотечение в ложе опухоли, что приводит к хронической постгеморрагической анемии [6]. Острые постгеморрагические анемии встречаются реже и наблюдаются в случае прорастания опухолевого конгломерата в крупный кровеносный сосуд или при спонтанном разрыве опухоли, что нередко бывает при гепатоцеллюлярном раке. И в том, и в другом случае всегда возникает дефицит железа, однако иногда причиной истощения запасов железа является нарушение его всасывания [7, 8]. Мальабсорбцию железа следует рассматривать у пациентов с дефицитом железа, которые не отвечают на лечение пероральными препаратами железа и/или в случае локализации опухоли в желудке, двенадцатиперстной или верхней части тощей кишки, т.е. в участках максимального всасывания железа [6].
Анемия может быть связана непосредственно с инфильтрацией опухолевых клеток (первичных или вторичных) в костный мозг. Из-за замещения нормальной кроветворной ткани костного мозга атипичными клетками и фиброзной тканью происходит угнетение гемопоэза с последующим снижением клеточности костного мозга и возможным развитием апластической анемии [7].
Иной патогенез наблюдается у пациентов с опухолями, сопровождающимися синдромом гиперспленизма и увеличением селезенки (например, при миело-, лимфопролиферативных заболеваниях, гепатоцеллюлярном раке) [9]. В данной ситуации из-за усиления фильтрующей способности селезенки происходит более быстрое разрушение эритроцитов, а зачастую и других ростков кроветворения. Содержание форменных элементов в периферической крови снижается, в то время как в костном мозге возникает компенсаторная гиперплазия с преобладанием незрелых предшественников эритроцитов и тромбоцитов.
Опухоль может также оказывать опосредованный эффект на систему кроветворения с помощью вырабатываемых в ответ провоспалительных цитокинов, подобно другим анемиям хронических заболеваний [10, 11]. Интенсивное взаимодействие между популяцией опухолевых клеток и иммунной системой приводит к продукции и высвобождению ряда цитокинов, таких как фактор некроза опухоли α (ФНО-α), интерфероны (ИФН-β, ИФН-ɣ), интерлейкины (ИЛ-1, -6) и другие хемокины [10, 11]. Как следствие – происходит повышение продукции в печени гормона гепсидина, являющегося центральным регулятором метаболизма железа [12]. Гепсидин относится к белкам острой фазы, поэтому его концентрация значительно возрастает при активном опухолевом процессе и воспалении. Связываясь с единственно известным белком – экспортером железа, ферропортином, он подавляет действие последнего. В ответ происходит ограничение абсорбции железа энтероцитами и блокирование его высвобождения в системный кровоток из клеток ретикулоэндотелиальной системы (макрофагов и гепатоцитов). Таким образом возникает функциональный (относительный) дефицит железа – состояние, при котором, запасы железа есть, но его недостаточно для нормального эритропоэза [12]. Прогрессирующее нарушение выхода железа из депо приводит к полному истощению запасов данного микроэлемента в организме – иными словами, развивается абсолютный дефицит железа (т.е. классическая железодефицитная анемия).
ФНО-α и ИЛ-1 также оказывают прямое ингибирующее действие на эритроидные клетки-предшественницы, разрушая мембрану эритроцитов, сокращают продолжительность их жизни со 120 дней до 60–90 дней [10, 11]. Те же цитокины снижают выработку эритропоэтина в почках – главного стимулятора эритропоэза, а также уменьшают чувствительность рецепторов эритроидных предшественников к эритропоэтину, тем самым увеличивают способность к их апоптозу. Кроме того, некоторые цитокины могут косвенно снижать экспрессию фактора, индуцируемого гипоксией-1 (HIF-1), который также стимулирует синтез эритропоэтина в почках [13].
Неадекватное взаимодействие между опухолью, системой гемостаза и иммунной системой может приводить к развитию анемии аутоиммунного генеза, возникающей в результате неконтролируемой выработки тепловых или холодовых аутоантител к поверхностным антигенам эритроцитов [6]. Аутоиммунная гемолитическая анемия является хорошо известным паранеопластическим синдромом при лимфопролиферативных заболеваниях, однако имеется ряд сообщений о такой ассоциации с солидными опухолями, особенно при почечно-клеточном раке и саркоме Капоши [14–16].
Другой механизм, при котором может происходить сокращение выживаемости эритроцитов, связан с редким, но крайне тяжелым феноменом – микроангиопатическим гемолизом, возникающим при опухоль-ассоциированном повреждении эндотелия сосудов (вторичной тромботической микроангиопатии) или ДВС-синдроме (синдроме диссеминированного внутрисосудистого свертывания) [17–19]. Тромботическая микроангиопатия представляет собой вариант паранеопластического синдрома, в основе которого лежит системный тромбоз микрососудов. «Потребление» тромбоцитов, идущих на образование тромба, приводит к развитию тромбоцитопении, вместе с тем постоянный контакт с тромбами, заполняющими просвет мелких сосудов, вызывает механическое повреждение эритроцитов и их разрушение, таким образом возникает микроангиопатическая гемолитическая анемия [17–19]. Отличительной особенностью мазка крови при такой анемии является наличие шистоцитов (фрагментированных эритроцитов) и отрицательный тест Кумбса. Случаи развития микроангиопатической гемолитической анемии в большей степени описаны для лимфомы, миеломы и некоторых солидных опухолей, таких как рак желудка, молочной железы, предстательной железы, легких, и опухоли без выявленной первичной локализации [17].
Осложнения противоопухолевого лечения
Токсические проявления противоопухолевой терапии считаются основным фактором, вызывающим или усугубляющим развитие анемии у онкологического пациента [20, 21]. Например, согласно ретроспективному анализу 210 пациентов с первичной опухолью центральной нервной системы, лучевая терапия в объеме краниоспинального облучения примерно в 30% случаев сопровождается развитием тяжелой гематологической токсичности [22]. Однако чаще всего анемия возникает при проведении противоопухолевой лекарственной терапии [23]. В целом частота и тяжесть развития химиоиндуцированной анемии зависят от лекарственного препарата и режима его дозирования [22]. Длительность терапии также имеет определенное значение. Так, согласно результатам крупномасштабного проспективного исследования ECAS, анемия любой степени тяжести после 1-го цикла встречается у 19,5% пациентов, после 5-го цикла – уже у 46,7% [1].
В основе патогенеза анемии, вызванной ионизирующим излучением или противоопухолевыми препаратами, лежит нарушение пролиферации и созревания клеток-предшественниц гемопоэза в костном мозге, а также повышенное разрушение эритроцитов в костном мозге и периферической крови [20, 21]. Тяжелые длительные тромбоцитопении, сопровождающиеся геморрагическим синдромом, также могут приводить к анемии. Помимо прямого воздействия на клетки кроветворения анемия может возникать как результат нефротоксического действия цитостатиков, подавляющего синтез гемопоэтических факторов роста, главным образом эритропоэтина [24]. Еще один потенциальный механизм связан с гастроинтестинальными осложнениями, такими как тошнота, рвота, диарея, стоматит. В случае их развития объективно снижается потребление пищи и как результат – возникает недостаток питательных веществ, витаминов и минералов, необходимых для адекватного эритропоэза [20].
В группе риска находятся онкологические пациенты, перенесшие операцию на ЖКТ. Подобные операции могут приводить к ахлоргидрии, демпинг-синдрому или синдрому слепой кишечной петли и вызывать нарушение всасывания железа [25, 26].
С другой стороны, удаление опухолей желудка (источника внутреннего фактора Касла) или терминальной части подвздошной кишки (места всасывания витамина B12) может приводить к дефициту витамина B12. Однако, поскольку его запасы в организме достаточно велики, а выведение происходит медленно, для развития значимого дефицита витамина B12 после такой операции могут потребоваться годы.
Коморбидные состояния
Существуют и другие причины развития анемии, не связанные с наличием неопластического процесса. Провоцировать или усугублять анемию могут любые сопутствующие заболевания, которые прямо или косвенно влияют на гемопоэз. К таким заболеваниям относятся хронические инфекционно-воспалительные и аутоиммунные заболевания (вызывают т.н. анемию хронических заболеваний), хроническая почечная недостаточность (критической является скорость клубочковой фильтрации – СКФ<60 мл/мин/1,73 м2), коагулопатии, наследственные заболевания (например, гемоглобинопатии), патология печени и другие заболевания, сопровождающиеся синдромом гиперспленизма, патология ЖКТ, гормональные нарушения (гипогонадизм, надпочечниковая недостаточность, гипер- или гипотиреоз), нутритивная недостаточность и т.д. [6, 27, 28].
Влияние анемии на результаты лечения
Основным последствием нарастающей анемии является тканевая гипоксия, ухудшается оксигенация и в опухолевой ткани. Экспериментально доказано, что локальная гипоксия опухоли приводит к перестройке активности генома, стимуляции неоангиогенеза и блокированию апоптоза, что провоцирует дальнейший рост опухоли [29]. Кроме того, в условиях гипоксии опухолевые клетки становятся менее чувствительными к воздействию цитостатиков и облучения [30, 31]. Так, на моделях фибросаркомы in vivo установлено снижение противоопухолевой активности до 6 раз, если опухолевые клетки были плохо насыщены кислородом [30].
В ряде работ отмечена убедительная корреляция между наличием анемии и эффективностью противоопухолевого лечения, а также прогнозом заболевания. Эта зависимость, в частности, подтверждена австрийскими коллегами у 424 пациенток, получавших адъювантную химиотерапию по поводу рака молочной железы [32]. Местный рецидив чаще возникал, если на фоне химиотерапии уровень Hb снижался ниже нормы (19,5 и 6,9% соответственно; р=0,0006). В целом у пациенток данной когорты риск развития локального рецидива в течение ближайших 5 лет после завершения лечения был в 2,95 раза выше (95% доверительный интервал [ДИ]: 1,4–6,2; р=0,004).
Для пациентов, проходящих курс лучевой терапии, пониженный уровень Hb также является негативным прогностическим фактором.
H. Frommhold et al. установили 2-кратное снижение 5-летней общей выживаемости среди пациентов с опухолями головы и шеи при условии наличия анемии (28,4 и 58,2% соответственно; p<0,0001) [33].
Несколько исследований также продемонстрировали связь между уровнем Hb и качеством жизни онкологических пациентов. Согласно исследованию ECAS, низкий соматический статус (ECOG≥2) имеет почти каждый четвертый (24,8%) пациент с анемией легкой степени тяжести и уже каждый второй (50,7%) – с анемией тяжелой степени [1]. Для таких пациентов даже незначительные отклонения от нормы в общем анализе крови могут приводить к серьезным последствиям, включая снижение толерантности к физическим нагрузкам, когнитивные расстройства и декомпенсацию сопутствующей патологии.
Диагностика
Анемия определяется как снижение концентрации Hb и в подавляющем большинстве случаев – числа эритроцитов в единице объема крови [6]. Отечественные и зарубежные онкологические сообщества, а также Всемирная организация здравоохранения (ВОЗ) выделяют разные пороговые значения уровня Hb в крови, позволяющие ставить диагноз анемии при ЗНО (табл. 1) [27, 34–36]. Отдельные пороговые значения разработаны для анемии, возникшей вследствие противоопухолевой лекарственной терапии. В данной ситуации используются критерии токсичности NCI CTCAE (National Cancer Institute Common Toxicity Criteria for Adverse Events), согласно которым анемией считается снижение концентрации Hb в крови ниже 130 г/л у мужчин и ниже 120 г/л у женщин [37].
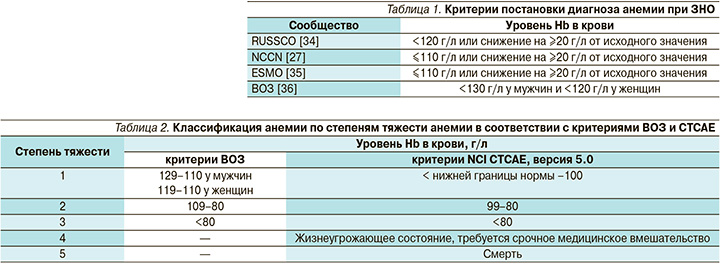
В зависимости от выраженности снижения концентрации Hb выделяют три степени тяжести анемии, согласно критериям ВОЗ, и пять степеней тяжести, согласно шкале токсичности NCI CTCAE (табл. 2) [36, 37].
Как ранее уже упоминалось, анемия при ЗНО может быть многофакторной, поэтому на этапе диагностического поиска необходимо в первую очередь определить патогенетический вариант анемии, т.е. основной механизм, провоцирующий снижение уровня Hb у данного пациента. Следующей задачей врача является распознавание патологического состояния или заболевания, которое могло привести к анемии. Установление причины развития анемии имеет решающее значение в выборе дальнейшей тактики, поскольку позволяет проводить патогенетическую терапию и одновременно воздействовать на основное заболевание.
При сборе анамнеза у онкологических пациентов с анемией необходимо уточнить характер и длительность предшествовавшей противоопухолевой терапии, семейный анамнез, а также наличие сопутствующей патологии.
Основным и первоочередным лабораторным методом диагностики анемии при ЗНО выступает общий анализ крови с микроскопией мазка. С его помощью анализируются такие показатели периферической крови, как Hb, гематокрит, эритроциты, эритроцитарные индексы (MCV, MCH, MCHC, RDW), ретикулоциты и ретикулоцитарный индекс, т.е. производится количественная и качественная оценка состояния эритроцитарного ростка.
Второй ключевой инструмент диагностики – биохимический анализ крови с обязательным определением маркеров метаболизма железа (сывороточное железо, общая железосвязывающая способность сыворотки – ОЖСС, ферритин, трансферрин), уровня витамина В12 и фолиевой кислоты (для исключения алиментарной анемии); концентрации креатинина и СКФ (для исключения почечной недостаточности); уровня С-реактивного белка (для исключения хронического воспаления).
Для уточнения происхождения анемии дополнительно может потребоваться выполнение следующих исследований [7, 27, 34, 38]:
- общий анализ мочи с микроскопией осадка, кал на скрытую кровь, эндоскопия, компьютерная томография при подозрении на скрытое кровотечение;
- проба Кумбса, ДВС-панель, определение концентрации гаптоглобина, непрямого билирубина, лактатдегидрогеназы при подозрении на гемолиз;
- исследование пунктата костного мозга с подсчетом миелограммы при подозрении на первичную или вторичную опухолевую инфильтрацию либо в случае отсутствия увеличения концентрации Hb в крови на фоне проводимой ее коррекции;
- определение концентрации эритропоэтина в крови при подозрении на поражение почек;
- анализы на гормоны щитовидной железы, надпочечников при подозрении на гормональные нарушения.
Для практикующего врача наиболее важна дифференциальная диагностика абсолютного дефицита железа (железодефицитной анемии) и функционального дефицита железа (анемии хронических заболеваний), что влияет на выбор метода лечения [27, 35, 38]. В табл. 3 приведены параметры, позволяющие дифференцировать эти патологические состояния.

Важным маркером дефицита железа является ферритин, представляющий собой основную форму депонированного железа [6, 7]. В то же время ферритин относится к белкам острой фазы, а значит, его уровень может повышаться при активном воспалительном процессе, при этом не коррелируя с истинными запасами железа в организме. Другие параметры феррокинетики, такие как трансферрин, ОЖСС, коэффициент насыщения трансферрина железом (отношение железа сыворотки к ОЖСС), отражают количество биологически доступного железа [6, 7]. Дефицит железа устанавливается при сатурации трансферрина менее 20% [35, 38]. Универсальных пороговых значений сывороточного ферритина как критерия дифференциальной диагностики дефицита железа нет. Например, в практических рекомендациях NCCN (National Comprehensive Cancer Network) абсолютный дефицит железа диагностируется при уровне ферритина менее 30 нг/мл, а согласно рекомендациям ESMO (European Society for Medical Oncology), пороговым значением является уровень в 100 нг/мл, как и при наличии воспалительного процесса [27, 35]. В целом, чем ниже показатели ферритина, тем выше вероятность истинного дефицита железа. Если показатели сатурации трансферрина и ферритина расходятся, приоритетным в постановке диагноза должно быть низкое значение уровня ферритина [27, 35, 38].
Лечение
Заместительные гемотрансфузии
Для пациентов, которым требуется немедленная коррекция анемии, заместительные гемотрансфузии остаются наиболее оптимальным вариантом. Переливание только 1 единицы эритроцитарной массы (300–450 мл) приводит к скорому повышению уровня Hb в среднем на 10 г/л и гематокрита –
на 3%, если нет продолжающегося активного кровотечения [27, 38, 39]. Однако гемотрансфузии дают кратковременный эффект и сопряжены с большим числом осложнений [27, 35, 39]. К ним относятся непосредственные посттрансфузионные осложнения (гемолитические, фебрильные негемолитические, аллергические реакции, острое поражение легких, гипотермия, волемическая перегрузка, цитратная интоксикация, ацидоз, гиперкалиемия) и отдаленные осложнения (гемолиз, реакция «трансплантат против хозяина», гемосидероз, трансмиссивные инфекции) [27, 38, 39]. Другим не менее серьезным осложнением является развитие эмболии. Доказано, что переливание эритроцитарной массы пациентам с онкологическим заболеванием ассоциировано с повышенным риском артериальных (относительный риск [ОР]=1,53; 95% ДИ: 1,46–1,61; р<0,001) и венозных тромбозов (ОР=1,60; 95% ДИ: 1,53–1,63; р<0,001), а также с повышенным риском внутрибольничного летального исхода (ОР=1,34; 95% ДИ: 1,29–1,38; р<0,001) [40]. В ряде исследований также сообщалось об увеличении частоты местных рецидивов и снижении общей выживаемости онкологических пациентов, перенесших трансфузии донорских компонентов крови перед оперативным вмешательством [41]. Для пациентов, получающих противоопухолевую лекарственную терапию, подобной доказательной базы пока нет.
Трансфузии эритроцитарной массы в первую очередь показаны при острой массивной кровопотере, сопровождающейся уменьшением объема циркулирующей крови менее 25–30%, Hb менее 70–80 г/л и гематокрита менее 25% [39]. В случае хронической анемии гемотрансфузии расцениваются как «последний рубеж» терапии, поскольку введение донорских эритроцитов может подавлять собственный эритропоэз реципиента [39]. Переливание эритроцитарной массы таким пациентам целесообразно проводить при уровне Hb менее 70–80 г/л или менее 90 г/л, если пациент страдает сердечно-сосудистыми заболеваниями, или при наличии выраженных симптомов вне зависимости от порогового уровня Hb [27, 38]. Однако главной задачей является устранение причины, вызвавшей анемию.
Необходимо принимать во внимание, что, хотя в 1 единице эритроцитарной массы содержится 200–278 мг железа, переливание эритроцитов не устраняет дефицита железа [27, 42]. Это связано с тем, что средний срок жизни перелитых эритроцитов составляет примерно 100–110 дней, поэтому железо, которое в конечном итоге будет фагоцитировано из перелитых эритроцитов, не сразу становится доступным для эритроцитоза [27, 42]. По этой причине перед гемотрансфузией необходимо определять показатели обмена железа и при необходимости добавлять соответствующие препараты после процедуры переливания крови [27, 38].
Стимуляторы эритропоэза
Стимуляторы эритропоэза наряду с гемотрансфузиями являются одним из вариантов лечения химиоиндуцированной анемии. Однако в отличие от переливания донорских эритроцитов эта группа препаратов обеспечивает медленный, но пролонгированный подъем уровня Hb и выход в кровь функционально полноценных эритроцитов [27]. Применение эритропоэтинов также позволяет достоверно снижать потребность в заместительных гемотрансфузиях (ОР=0,65; 95% ДИ: 0,62–0,68) [43].
Существенным недостатком применения стимуляторов эритропоэза помимо аллергических реакций является потенциальный риск осложнений со стороны сердечно-сосудистой системы. По данным кокрейновского обзора, терапия эритропоэтином или дарбэпоэтином (в сочетании с гемотрансфузиями при необходимости) у онкологических пациентов увеличивает риск развития тромбоэмболических осложнений в 1,5 раза (ОР=1,52; 95% ДИ: 1,34–1,74; 57 исследований, n=15,498), артериальной гипертензии – в 1,3 раза (ОР=1,30; 95% ДИ: 1,08–1,56; 31 исследование, n=7,228), тромбоцитопении/кровотечения – в 1,2 раза (ОР=1,21; 95% ДИ: 1,04–1,42; 21 исследование, n=4,507) [43]. Исходя из вышеизложенного, пациентам с исходно высоким риском тромбообразования следует с осторожностью назначать эритропоэз-стимулирующие препараты. Эффективность ацетилсалициловой кислоты или антикоагулянтов в качестве профилактики тромбоэмболических осложнений в настоящее время не доказана.
Другим неблагоприятным побочным эффектом применения стимуляторов эритропоэза является развитие парциальной красноклеточной аплазии, характеризующейся потерей эритробластов в костном мозге вследствие образования нейтрализующих антител к эритропоэтину. Этот редкий феномен описан лишь среди пациентов с хронической почечной недостаточностью [44]. Тем не менее, если у пациента наблюдается внезапная потеря терапевтического ответа на введение стимуляторов эритропоэза и при этом развивается тяжелая нормоцитарная анемия с низким количеством ретикулоцитов, необходимо, в частности, исключать парциальную красноклеточную аплазию. При подтверждении диагноза дальнейшую терапию эритропоэтинами отменяют, смена на другой стимулятор также не допускается ввиду возможного перекрестного реагирования с нейтролизующими антителами.
Дискутабельным остается вопрос о влиянии стимуляторов эритропоэза на прогрессирование опухолевого процесса. В вышеупомянутом кокрейновском обзоре показано увеличение риска смертности на 17% (95% ДИ: 1,06–1,29; 70 исследований, n=15 935), особенно при достижении целевого уровня Hb более 120 г/л [43]. В других исследованиях не было подтверждено ухудшение показателей общей или безрецидивной выживаемости при применении препаратов этой группы [45–47].
Единственным показанием к назначению эритропоэз-стимулирующих препаратов является химиоиндуцированная анемия ≥2-й степени тяжести при условии отсутствия абсолютного дефицита железа [27, 34, 35, 38]. Препараты этой группы назначают только пациентам с немиелоидными ЗНО, получающим миелосупрессивную химиотерапию с паллиативной целью [48, 49]. При этом нет четких инструкций для пациентов, получающих таргетные препараты, в частности сунитиниб, эрлотиниб или трастузумаб, которые также часто способствуют развитию анемии.
В связи с неоднозначными данными о возможном потенцировании опухолевого роста при применении эритропоэз-стимулирующих препаратов следует избегать их назначения пациентам, получающим миелосупрессивную терапию по поводу потенциально излечимых новообразований, в т.ч. при проведении (нео)адъювантной химиотерапии [27, 35, 48, 49]. Хотя до настоящего времени рандомизированных клинических исследований, в которых пациенты были бы разделены на группы в зависимости от цели лечения, еще не проводилось.
Следующей категории пациентов также не рекомендуется назначение стимуляторов эритропоэза: 1) при высоком уровне сывороточного эритропоэтина; 2) при уровне Hb более 100 г/л; 3) пациентам, получающим гормональную терапию, биологические препараты либо лучевую терапию без дополнительной миелосупрессивной химиотерапии; 4) пациентам для лечения анемии, вызванной другими факторами, такими как дефицит витамина В12 или фолиевой кислоты, гемолиз, желудочно-кишечное кровотечение или кровотечение вследствие тромбоцитопении; 5) пациентам, требующим немедленной коррекции анемии путем заместительной гемотрансфузии [27, 35, 48, 49].
Главной целью терапии стимуляторами эритропоэза является предотвращение гемотрансфузий путем повышения уровня Hb [27, 35]. Если спустя 2 недели с момента старта лечения уровень Hb увеличился на ≥1 г/л или достигнут целевой уровень Hb, при котором нет необходимости в гемотрансфузии, дозу стимуляторов следует снижать (на 25% при терапии эпоэтином и на 40% при терапии дарбэпоэтином) [27, 35, 38, 48, 49]. В отсутствие положительного эффекта (уровень Hb увеличился <1 г/л или остается в пределах <100 г/л) в течение 4 недель терапии эпоэтином-α или 6 недель терапии дарбэпоэтином-α дозу необходимо увеличивать, дополнительно необходимо исключать иные причины, способствующие развитию анемии, включая дефицит железа [27]. Стоит отметить, что почти у трети пациентов развивается резистентность к эритропоэтинам [50, 51]. Одна из причин может быть связана с наличием у пациента анемии другой этиологии [42, 51]. Другой возможной причиной является развитие функционального дефицита железа на фоне лечения эритропоэтинами, когда из-за повышенной продукции эритроцитов возникает дисбаланс между потребностью организма в железе и его обеспечением [27, 48, 49]. В этой ситуации целесообразно добавить к терапии стимуляторами эритропоэза препараты железа [27]. Между тем высокая частота терапевтического ответа наблюдается только при применении парентеральных форм железа (ОР=1,29; 95% ДИ: 1,13–1,48; р=0,0001) [51]. Наконец, если нет эффекта в течение 8 недель даже при добавлении препаратов железа, а потребность в переливании крови сохраняется, терапия стимуляторами эритропоэза должна быть полностью прекращена [27, 35, 38, 48, 49]. Убедительных данных в пользу смены препарата в случае недостаточного ответа нет [35]. Стимуляцию эритропоэза также необходимо отменить после завершения курса химиотерапии (обычно через 6–8 недель после последнего цикла, когда восстановится функция костного мозга) [27, 42].
Железосодержащие препараты
Железосодержащие препараты в монотерапии рекомендуется назначать при абсолютном дефиците железа [27, 35, 38]. Лучший гемопоэтический ответ и сокращение потребности в гемотрансфузиях (ОР=0,72; 95% ДИ: 0,55–0,95) наблюдаются при применении инфузий железа по сравнению с таблетированными формами [52]. Парентеральные формы железа считают методом выбора при состояниях, не позволяющих принимать препараты внутрь (например, при тошноте, рвоте, анорексии) или сопровождающихся нарушением всасывания железа (например, при воспалительных заболеваниях ЖКТ или оперативных вмешательствах), а также при плохой непереносимости пероральных форм железа либо при необходимости быстрого восполнения запасов железа и увеличения уровня Hb [27, 38, 53].
Считается, что уровень Hb в крови должен повышаться после 4 недель лечения. Если пациент сначала получает пероральный препарат железа, а ожидаемый ответ не наблюдается через 4 недели, рекомендуется перейти на парентеральное введение железа.
В отсутствие эффекта после внутривенных инфузий железа в течение 4 недель необходимо обследовать пациента на предмет функционального дефицита железа. Для пациентов с функциональным дефицитом железа следует рассматривать возможность внутривенного введения железа в сочетании со стимуляторами эритропоэза [27, 35, 38].
Терапия железосодержащими препаратами не лишена рисков [27, 35]. Помимо известных аллергических и диспепсических расстройств есть ограниченные данные о том, что парентеральные формы железа могут повышать восприимчивость к бактериальной инфекции [54, 55]. Поэтому рекомендуется избегать внутривенного введения железа пациентам с активным или хроническим воспалительным процессом, нейтропенией или выраженной иммуносупрессией [27, 38]. Ряд авторов не рекомендуют вводить железо в дни проведения химиотерапии (преимущественно обладающей кардиотоксическим эффектом) из-за гипотетического риска межлекарственного взаимодействия [27, 34, 38, 42]. Также недопустимо одновременное применение парентеральных и пероральных препаратов железа из-за уменьшения абсорбции последних [27, 42].
В целом терапия железосодержащими препаратами считается относительно безопасным методом коррекции анемии при наличии злокачественного процесса. Хотя долгосрочные побочные эффекты у онкологических пациентов до конца не установлены, железосодержащие препараты не повышают риска развития тромбоэмболии или иных осложнений со стороны сердечно-сосудистой системы [27, 38]. Нет и убедительных клинических данных, свидетельствующих о стимуляции опухолевого роста на фоне терапии препаратами железа [35, 38].
Альтернативные средства лечения анемии у онкологических пациентов зависят от ее патогенетического варианта. Подробное описание методов коррекции анемии другой этиологии выходит за рамки данного обзора.
Заключение
Принято считать, что развитие анемии у пациентов, страдающих ЗНО, является неизбежным процессом.
С другой стороны, проблема анемии и ее значение для пациента явно недооценены. Активное использование агрессивных методов лечения способно ухудшать качество жизни пациентов и поэтому требует активной поддерживающей терапии. При выборе метода лечения анемии у онкологического пациента в первую очередь следует учитывать этиологический фактор и лишь затем – степень снижения концентрации Hb в крови. Немаловажную роль играет коморбидный статус пациента, что в некоторых ситуациях может ограничивать применение заместительных гемотрансфузий или стимуляторов эритропоэза. Врачи также должны быть осведомлены об их возможных серьезных побочных эффектах. Правильная и своевременная коррекция анемии позволяет улучшать функциональный статус пациента и достигать более высоких результатов противоопухолевого лечения.



