Введение
По данным GLOBOCAN, в 2020 г. рак желудка (РЖ) диагностирован у 1,1 млн человек и 770 тыс. больных умерли от этого заболевания. Смертность составила 8% от всех смертей, связанных со злокачественными новообразованиями [1]. Ожидается, что к 2040 г. ежегодное количество заболевших увеличится до 1,8 млн человек, умерших – до 1,3 млн [2].
На момент постановки диагноза от 30 до 44% пациентов уже имеют отдаленные метастазы РЖ [3, 4]. По данным Qiu et al. [3], частота выявления метастазов в печени составляет 41,3%, в легких – 14,4%, в костях – 12,4%, в головном мозге – 1,9%.
Основным методом лечения, направленным на излечения больных РЖ, по-прежнему остается хирургический. При наличии отдаленных метастазов ведущую роль играет противоопухолевая лекарственная терапия, применение хирургического метода ограничено ликвидацией таких осложнений опухолевого процесса, как перфорация, кровотечение, обструкция желудочно-кишечного тракта [4].
Несмотря на современные достижения лекарственной терапии, прогноз при IV стадии заболевания остается неблагоприятным, медиана общей выживаемости (МОВ) пациентов составляет 13–17 месяцев [5, 6]. Внедрение таргетных препаратов и ингибиторов контрольных точек привело к умеренному увеличению выживаемости больных диссеминированным РЖ, в связи с чем возродился интерес к изучению возможности увеличения продолжительности жизни пациентов за счет комбинации лекарственной терапии с хирургическим лечением.
В литературе появились такие понятия, как «conversion surgery» (конверсионная хирургия) и «conversion therapy» (конверсионная терапия/конверсионное лечение), предложенные Yoshida [7]. Они отражают разную последовательность применения оперативного вмешательства и химио-терапии (ХТ). В первом случае сначала выполняют условно «радикальное» хирургическое вмешательство в объеме R0 с полным удалением всех выявленных очагов опухоли, на втором этапе проводят ХТ. Этот подход возможен у пациентов с т.н. олигометастатическим РЖ [8]. «Сonversion therapy» подразумевает первоначальное лекарственное противоопухолевое лечение пациентов с исходно неоперабельным диссеминированным РЖ для перевода опухолевого процесса в т.н. резектабельное состояние с возможностью последующего полного хирургического удаления и первичной опухоли и имеющихся метастазов (R0). Следует отметить, что в последнее десятилетие в литературе часто смешивают понятия «конверсионная хирургия» и «конверсионная терапия/конверсионное лечение», подразумевая выполнение оперативного пособия R0 после проведения лекарственной терапии.
В представленном ниже тексте термин «конверсионное лечение» использован только при лекарственной терапии с последующим хирургическим лечением с намерением полного удаления всех выявленных очагов РЖ.
Влияние паллиативной гастрэктомии на выживаемость больных РЖ IV стадии
Роль паллиативной гастрэктомии (ПГЭ) при РЖ IV стадии была оценена в нескольких небольших исследованиях с ограниченным числом пациентов и в ряде ретроспективных анализов, давших противоречивые результаты. Так, Schmidt et al., Kokkola et al., Gold et al. [9–11] не выявили позитивного влияния ПГЭ на выживаемость пациентов с РЖ IV стадии. В то же время ряд исследователей показали, что хирургическое вмешательство с намерением полного удаления всех проявлений опухоли (R0) увеличило 3-летнюю выживаемость пациентов до 32% [12–20] (данные представлены в табл. 1).

В ряде исследований на основе многофакторного регрессионного анализа выделены факторы благоприятного прогноза. Так, в исследовании Hartgrink et al. хирургическое вмешательство значимо улучшило ОВ пациентов только с одной зоной метастатического поражения до 10,5 месяцев. У остальных больных данный показатель не превысил 6,7 месяца (p=0,034). Максимальный эффект от оперативного лечения отмечен у пациентов моложе 70 лет, имевших только одну зону метастазирования [13]. Исследования японской клинической онкологической группы также показали, что к независимым факторам благоприятного прогноза относятся небольшое количество зон метастатического поражения, удовлетворительный функциональный статус пациента и макроскопически нескиррозный тип опухоли [21].
Dittmar et al. к независимым факторам прогноза выживаемости больных РЖ после ПГЭ отнесли проведение ХТ и вовлечение в опухолевый процесс только параортальных лимфоузлов [15]. В исследовании Lin et al. 5-летняя выживаемость больных, которым после операции провели ХТ, составила 8,9% и статистически значимо превысила аналогичный показатель пациентов без послеоперационной ХТ (1,4%; р=0,037) [20].
Chiu et al. показали, что выполнение ПГЭ на первом этапа лечения является фактором благоприятного прогноза ОВ. Однако при повышенном уровне раково-эмбрионального антигена (РЭА) или ракового антигена CA 19-9 преимущества паллиативной резекции желудка нет [17]. Позитивное влияние ПГЭ на ОВ больных получено в мета-анализах Sun et al. [22] и Lasithiotakis et al. [23].
Результаты проведенных ретроспективных исследований позволили выделить ряд показаний к оперативному лечению больных РЖ IV стадии [24]:
- удовлетворительное общее состояние пациента [21];
- наличие только одной зоны отдаленного метастазирования [12, 15, 21] или изолированных исходно резектабельных метастазов в печени [18, 19];
- обязательная комбинация оперативного лечения с ХТ [15, 20];
- чувствительность опухоли к ХТ [18];
- уровень опухолевых маркеров РЭА и СА 19,9 в пределах референсных значений на момент оперативного вмешательства [17].
Однако данные единственного проспективного многоцентрового контролируемого рандомизированного исследования REGATTA [25] не подтвердили выводов ретроспективных анализов.
В исследование REGATTA были включены 175 больных диссеминированным РЖ с одним фактором неоперабельности в виде ограниченного метастатического поражения либо печени, либо брюшины, либо пара-аортальных лимфатических узлов групп 16а1/16b2. После рандомизированного выбора тактики лечения пациентам 1-й группы (n=86) провели только ХТ цисплатином+S1, пациентам 2-й группы (n=89) – гастрэктомию с лимфодиссекцией в объеме D1 без резекции метастатических очагов с последующей ХТ в том же режиме. Первичной конечной точкой являлась ОВ. Результаты исследования опубликованы в 2016 г. МОВ больных, получивших только ХТ, составила 16,6 месяца и статистически значимо не отличалась от аналогичного показателя в группе комбинированного лечения (14,3 месяца; р=0,07). [25].
Итоги исследования REGATTA вызвали активную дискуссию в научной среде. Критикуя исследование, ряд авторов обращали внимание на гетерогенность популяции больных, включенных в исследование, неадекватность объема лимфодиссекции (D1), отсутствие одновременного удаления резектабельных отдаленных метастазов, т.е. R0 операции, включение в исследование только азиатской популяции больных.
Для определения возможности экст-раполяции результатов исследования REGATTA на европейскую популяцию Warschkow et al. [26] провели ретроспективный анализ данных лечения 7026 первичных больных диссеминированным РЖ, выделенных из нацио-нальной базы по раку (The National Cancer Database, NCDB) за 2006–2012 гг., согласно критериям отбора больных, в исследование REGATTA. МОВ пациентов, получивших оперативное лечение с последующей ХТ, составила 13,9 месяца и с высокой степенью достоверности превысила аналогичный показатель пациентов, получивших только ХТ (7,9 месяца; p<0,001). Двугодичная выживаемость составила соответственно 25,4 и 13,1%. Анализ методом «псевдорандомизации» (propensity score matching, PSM) дал такие же результаты.
Kamarajah et al. [27] отобрали из национальной базы данных по раку (NCDB) за 2010–2015 гг. 19 411 пациентов с отдаленными метастазами на момент постановки диагноза. После ПГЭ МОВ (12,8 месяца) и 5-летняя выживаемость (14%) больных были существенно выше, чем после проведения одной ХТ (9,5 месяцев; p<0,001, и соответственно 3%; p<0,001). Учтя несовпадение полученных результатов с данными исследования REGATTA, авторы сделали вывод о необходимости дополнительных рандомизированных исследований только на европейской популяции.
Результаты исследований, опубликованные в 2019–2022 гг., подтверждают, что ПГЭ значимо улучшает выживаемость пациентов и может служить благоприятным прогностическим фактором [28–32]. Так, ретроспективный анализ Zheng et al. [32] показал, что МОВ оперированных больных и пациентов, получавших только ХТ, составили соответственно 12,0 и 9,0 месяцев (p=0,020). Проведенный авторами мета-анализ 10 исследований подтвердил полученный результат: ПГЭ с последующей ХТ способствовала значимому (p<0,001) улучшению общей выживаемости по сравнению с одной только ХТ.
Существенным недостатком практически всех представленных в настоящее время в литературе работ считались их ретроспективный и одноцентровый характер, отсутствие рандомизации, охват значительного периода времени без учета совершенствования методов диагностики, хирургического вмешательства, анестезиологии и реаниматологии, а также прогресса в разработке новых режимов лекарственной противоопухолевой терапии.
Несмотря на недостатки, REGATTA в настоящее время является единственным проспективным рандомизированным многоцентровым исследованием и служит основой для определения лечебной тактики для больных РЖ IV стадии с ограниченным метастазированием. Полученные результаты ясно показали, что ПГЭ с частичным удалением опухолевых проявлений (в объеме R2) и последующей ХТ не увеличивает продолжительности жизни пациентов и нецелесообразна. С одной стороны, такая операция изначально носит паллиативный циторедуктивный характер, способствует уменьшению опухолевой нагрузки на организм, что теоретически может повышать эффективность последующей ХТ и превентивно устранять такие возможные осложнения РЖ, как обструкция, кровотечение и перфорация. С другой стороны, хирургическое вмешательство, подавляя иммунитет и высвобождая факторы воспаления (VEGF, IL-1β, IL-6, MCP-1 и TGC-β), может активизировать рост и дальнейшее метастазирование оставшихся опухолевых очагов [33–35]. Операция и ее потенциальные осложнения могут отодвигать сроки начала эффективной системной терапии, способствовать снижению толерантности к ней и ухудшать прогноз заболевания. Кроме того, выполнение ПГЭ на первом этапе лечения при высокой биологической гетерогенности РЖ не позволяет выделять когорту пациентов с быстрым прогрессированием болезни, которым именно раннее начало ХТ могло бы принести максимальную пользу.
Показав нецелесообразность выполнения ПГЭ в объеме R2 на первом этапе лечения больных олигометастатическим РЖ IV стадии, исследование REGATTA не ответило на вопрос о целесообразности выполнения R0-операций с последующей ХТ, который все еще остается предметом дискуссии [36].
Влияние конверсионного лечения на выживаемость больных РЖ IV стадии
Непрерывный прогресс противоопухолевой терапии с внедрением новых препаратов и комбинаций [5, 37–39] позволил не только повысить эффективность самого лекарственного лечения, но и надеяться на хорошие результаты его комбинации с хирургическим вмешательством, что будет способствовать увеличению выживаемости больных диссеминированным РЖ и в отдельных случаях даже их излечению [40].
Основным направлением современных исследований в этой области является конверсионное лечение – проведение ХТ с последующим хирургическим вмешательством при возможности выполнения операции в объеме R0, которое при РЖ находится еще на начальном этапе развития [36]. Для достижения оптимального результата планирование и проведение конверсионного лечения на всех этапах требуют обязательного обсуждения на мультидисциплинарном консилиуме с участием хирургов, химиотерапевтов, радиотерапевтов, рентгенологов, морфологов.
В табл. 2 представлены основные результаты немногочисленных исследований конверсионного лечения больных диссеминированным РЖ с различными факторами неоперабельности. К сожалению, практически все исследования являются ретроспективными одноцентровыми и включают небольшое число больных, что свидетельствует о редкости применения данной стратегии при РЖ IV стадии.
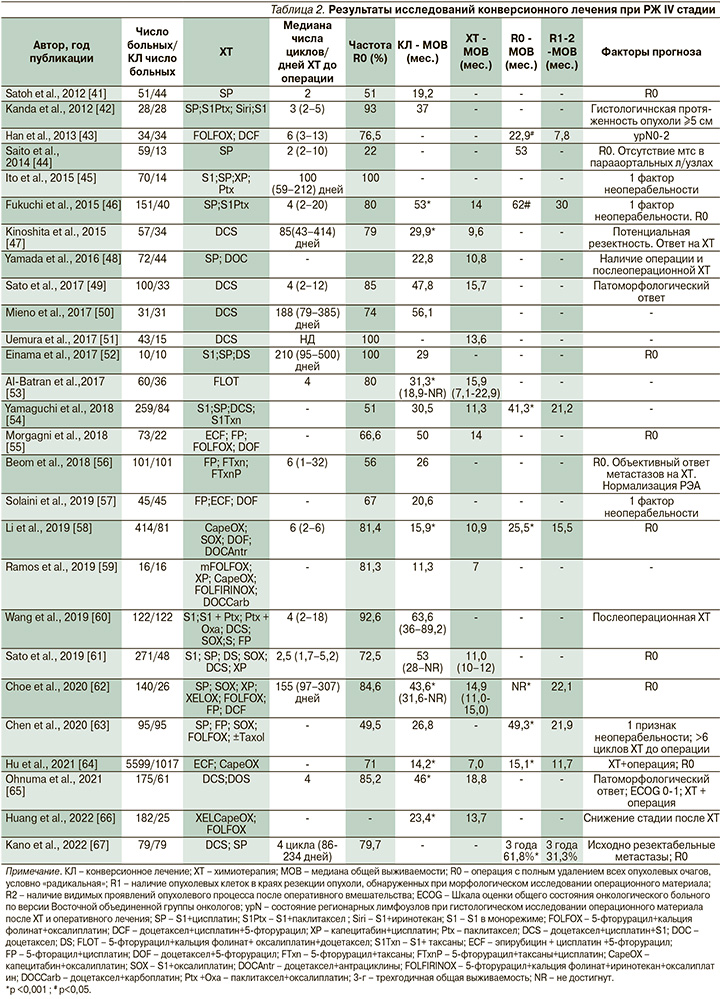
В 2017 г. опубликованы результаты пока единственного проспективного многоцентрового исследования II фазы «AIO-FLOT3» с участием 252 пациентов с местнораспространенной или метастатической аденокарциномой желудка, или кардиоэзофагеального перехода [53]. В зависимости от распространенности процесса больных разделяли на три группы. У пациентов группы А исходно диагностирован резектабельный опухолевый процесс, группы В – олигометастатический процесс и группы С – РЖ с обширным метастазированием. Пациентам группы А проводили периоперационную ХТ в режиме FLOT (4 цикла до и 4 цикла после операции) в комбинации с радикальным оперативным вмешательством.
Пациенты группы В после проведения 4 циклов в режиме FLOT проходили полное обследование для рестадирования РЖ и в случае возможности удаления всех опухолевых проявлений (R0) выполняли операцию с последующими 4 циклами режима FLOT. Пациентам группы С проводили только ХТ в режиме FLOT. В среднем больные получили по 8 циклов ХТ (от 1 до 15).
МОВ и частота объективного ответа на ХТ в группе В составили соответственно 22,9 месяца и 60%, статистически значимо превысив аналогичные показатели группы С (10,7 месяца; p<0,001, и 43,3%; p=0,04).
У 10% больных группы В зарегистрирована полная регрессия опухоли; 60% прооперированы, МОВ у них достигла 31,3 месяца, в то время как у остальных была в 2 раза ниже (15,9 месяца). Максимальный эффект конверсионного лечения отмечен у пациентов с изолированными метастазами в забрюшинных лимфатических узлах [53].
В 2023 г. опубликованы результаты пока также единственного проспективного исследования III фазы Neo-REGATTA [68], в которое были включены 73 больных РЖ IV стадии с олигометастазами. Всем проведено 4 цикла индукционной ХТ в режиме DOS, после чего в отсутствие прогрессирования пациентов включали в протокол. Согласно протоколу, пациентам либо выполняли операцию с последующим проведением 4 курсов в режиме DOS, либо продолжали ХТ до 8 циклов с последующей поддерживающей терапией S1 до прогрессирования процесса. После проведения первых 4 циклов ХТ у 13 пациентов выявлено прогрессирование заболевания. В финальный анализ результатов лечения с оценкой ОВ, выживаемости без прогрессирования (ВБП) и переносимости включены 60 человек, 35 из них выполнена операция, 25 получили только ХТ. После хирургического вмешательства 51,4% больных не смогли перенести интенсивную ХТ в полном объеме. Медиана периода наблюдения составила 30 месяцев, медиана ВБП и МОВ больных, получивших конверсионное лечение, не достигнута, но с высокой степенью достоверности (p<0,0001) выше, чем в группе ХТ: 9,0 и 18,0 месяцев соответственно. Одно- и двугодичная ВБП и ОВ в группе ХТ составили соответственно 35,3%, 29,1 и 73,1, 25,8%, в группе комбинированного лечения – 84,7%, 77,1 и 96,9, 81,7%.
Анализ результатов лечения, проведенный методом «псевдорандомизации», подтвердил значимое увеличение выживаемости после конверсионного лечения по сравнению с ХТ без операции. В группе пациентов, получавших конверсионное лечение, МОВ и медиана ВБП не достигнуты, в группе пациентов, получавших ХТ, показатели составили соответственно 20 и 10 месяцев.
Результаты мета-анализа 23 исследований, опубликованные Du et al. [69] в 2019 г., показали, что конверсионное лечение статистически значимо повышает 1- и 3-годичную выживаемость больных РЖ по сравнению с одной только ХТ и оперативным лечением в объеме R1–2 (p<0,001).
В 2021 г. Desiderio et al. [70] представил данные наиболее крупного сравнительного мета-анализа результатов лечения 16 596 больных РЖ IV стадии, включенных в базу данных SEER в 2004–2015 гг. Медиана OВ пациентов, получивших ХТ с последующей операцией, составила 15 месяцев и статистически значимо (р<0,001) превысила аналогичный показатель других групп больных: перенесших хирургическое вмешательство с послеоперационной ХТ (13 месяцев), получивших только хирургическое лечение в объеме резекции желудка (6 месяцев) и только одну ХТ (7 месяцев). Практически аналогичная выживаемость больных после одной ХТ и только гастрэктомии свидетельствует о нецелесообразности применения одного хирургического лечения при РЖ IV стадии.
В качестве прогностического фактора Desiderio et al. [70] впервые выделили год постановки диагноза и показали различия между 2004–2006-м и 2011–2015 гг. С течением времени прогресс в ХТ РЖ IV стадии и совершенствование хирургических методик привели к тому, что частота назначения системного лечения выросла с 18,8 до 48,8%, а частота конверсионного лечения увеличилась с 10,9 до 59,1% при снижении частоты выполнения ПГЭ на первом этапе лечения с 34,4 до 28,3%.
Обобщая результаты своего исследования, Desiderio et al. [70] пришли к выводу: оптимальной стратегией лечения больных РЖ IV стадии является комбинация ХТ с последующим оперативным лечением. Авторы не смогли выявить четкие критерии идентификации подгрупп пациентов по количеству и локализации метастазов вследствие того, что текущие исследования не выявили четких корреляций, позволяющих обобщить результаты по этим факторам. Таким образом, влияние этих параметров не может быть строго предопределено: скорее они должны оцениваться индивидуально [70].
Критерии отбора пациентов для конверсионного лечения
Конверсионное лечение показано далеко не всем больным диссеминированным РЖ. Поэтому необходимо четко обозначить критерии отбора, обеспечивающие максимальный эффект хирургического лечения с учетом не только его потенциальных рисков и снижения качества жизни в послеоперационном периоде, но и возможности стимуляции роста опухоли и снижения толерантности к последующей ХТ [40].
Для оценки возможности проведения конверсионного лечения уже на этапе первичной диагностики Yoshida et al. [7] предложили в 2016 г. классификацию РЖ IV стадии с учетом его гетерогенности и биологических характеристик. В зависимости от распространенности опухолевого процесса и локализации метастазов выделено четыре категории больных. Первая из них включает пациентов c потенциально операбельным опухолевым процессом (резектабельная первичная опухоль и ее метастазы, в частности солитарный метастаз в печени менее 5 см в диаметре без инвазии крупных сосудов или метастазы в парааортальных лимфоузлах групп 16a2 и/или 16b1, а также наличие опухолевых клеток в смывах с брюшины [cyt+]). Авторы считают, что в этих случаях операция с полным удалением опухолевых проявлений (R0) может быть выполнена вне зависимости от ответа на ХТ, если на фоне лечения не выявлены новые метастазы или признаки, свидетельствующие о нерезектабельности. Однако, как показано Li et al. [58], выполнение оперативного вмешательства на фоне прогрессирования болезни ухудшает прогноз. Для первой категории пациентов Yoshida et al. [7] допускают начало лечения с условно-радикальной операции (R0) с последующей ХТ до прогрессирования болезни или неприемлемой токсичности, однако, как показано выше, подобная лечебная стратегия не считается оптимальной. Так, Sakamoto et al. [85] выявили внутрипеченочный рецидив метастазов у 62% пациентов в течение 2 лет после операции R0 по поводу РЖ с единичными метастазами в печени, что свидетельствовало о наличии оккультных метастазов уже на момент хирургического лечения.
В исследовании Neo-REGATTA у 13 из 73 больных после проведения 4 циклов предоперационной ХТ выявлено прогрессирование процесса, что позволило избежать бессмысленной операции [68]. В тех случаях, когда cyt+ является единственным проявлением отдаленного метастазирования, операцию R0 следует проводить только после ХТ с достижением полного ответа (cyt0), доказанного повторной лапароскопией с морфологическим исследованием смывов с брюшины, т.е. после конверсии cyt+ в cyt0.
Во вторую категорию объединены пациенты c погранично операбельным процессом без метастазов по брюшине, но с погранично резектабельными или нерезектабельными (технически или с онкологических позиций) отдаленными метастазами, например солитарным метастазом в печени более 5 см в диаметре, или вовлечением в опухолевый процесс печеночной и/или воротной вены при наличии более 2 метастазов в печени или метастазов в забрюшинных лимфатических узлах, не относящихся к группам 16a2/b1, а также метастазов в отдаленных лимфатических узлах (медиастинальные, надключичные, подмышечные) или во внутренних органах. В этих случаях первым этапом лечения должна быть лекарственная терапия. Оперативное лечение может быть выполнено только после объективного ответа опухоли на системное лечение при отсутствии новых метастатических очагов и наличии возможности выполнить операцию в объеме R0. После хирургического лечения рекомендуется продолжить ХТ до прогрессирования процесса или неприемлемой токсичности.
Третью категорию составляют пациенты с макроскопически определяемой диссеминацией по брюшине, не имеющие других отдаленных метастазов. Им рекомендована интенсивная лекарственная терапия с последующей повторной лапароскопией. Последующее удаление первичной опухоли может быть выполнено только в тех случаях, когда лапароскопия подтверждает объективный ответ на системное лечение и установлена полная резорбция метастазов по брюшине с отсутствием опухолевых клеток в смывах с брюшины. В настоящее время ведутся активные поиски методов, повышающих эффективность ХТ у данной категории пациентов. Изучается комбинация системной ХТ с методами местного воздействия: внутрибрюшинной гипертермической ХТ, аэрозольной внутрибрюшинной ХТ под давлением, интраперитонеальной ХТ, однако убедительных доказательств их позитивного влияния на выживаемость еще не получено [36, 86].
К четвертой категории рекомендовано относить пациентов с макроскопически определяемыми метастазами по брюшине и другими отдаленными метастазами.
По мнению Yoshida et al. [7], поскольку целью конверсионного лечения является выполнение хирургического вмешательства в объеме R0, максимальное количество кандидатов можно найти в первой категории, немного меньше – во второй и минимум – в третьей. Пациенты, отнесенные к 4-й категории, почти не имеют шансов на конверсионное лечение.
Практическое применение данной классификации прослеживается в ряде работ последних лет.
В 2017 г. Yamaguchi et al. [54] представили ретроспективный анализ результатов лечения 259 больных диссеминированным РЖ, из которых 84 были оперированы. Пациенты были разделены на категории в соответствии с классификацией Yoshida et al. В первую категорию включены 9 человек, во вторую – 135, в третью – 31 и в четвертую – 84. МОВ всех 259 пациентов составила 15,1 месяца, получивших конверсионное лечение (n=84) – 30,5, после выполнения операции в объеме R0 – 41,3 месяца. Последний показатель был статистически значимо больше, чем у оперированных в объеме R1/R2 (21,2 месяца). В результате проведения одной только ХТ МОВ составила лишь 11,3 месяца. Вне зависимости от характера лечения МОВ пациентов 1-й, 2-й, 3-й и 4-й категорий составили соответственно 26,3, 14,8, 22,0 и 12,9 месяца. Частота выполнения конверсионного лечения пациентов 1-й категории достигла 77,8%, в категории 2 составила 31,1%, в категории 3 – 51,6%, в категории 4 – 22,6%. Почти во всех категориях МОВ пациентов, получивших оперативное лечение, была достоверно выше, чем у неоперированных больных. В категории 1 она составила соответственно 28,3 и 5,8 месяца (p=0,0019), в категории 2 – 30,5 и 11,0 (p <0,0001), в категории 3 – 31,0 и 18,5 (p=0,0839), в категории 4 – 24,7 и 10,0 месяцев (p<0,0001).
Ohnuma et al. [65] в 2021 г. опубликовали результаты ретроспективного анализа результатов лечения 175 пациентов с диссеминированным РЖ, из которых 114 получили только ХТ, 61 – конверсионное лечение. Распределив больных в соответствии с классификацией Yoshida et al., авторы также выявили преимущество конверсионного лечения по сравнению с одной только ХТ во всех категориях больных, за исключением категории 3 (категория 1: отношение рисков (ОР)=0,13, p<0,001; категория 2: ОР=0,41; p=0,008; категории 3: ОР 0,69; p=0,58; категория 4: ОР=0,13, p=0,003). Однако во всей подгруппе пациентов с перитонеальными метастазами конверсионное лечение способствовало значимому (ОР=0,38; p=0,04) увеличению МОВ (43,3 месяца) по сравнению с одной ХТ (14,3 месяца).
По данным Kano et al. [67], частота выполнения операции R0 больным 1-й (n=23), 2-й (n=17), 3-й (n=33) и 4-й (n=6) категорий составила соответственно 100, 94,1, 60,6, и 66,7%; 3-годичная выживаемость пациентов категории 1 была существенно выше, чем у больных категорий 2–4: 78,3 и 44,5% (p<0,001); 3-годичная выживаемость была значимо выше после операции R0 по сравнению с R1–2: 61,8 и 31,3% соответственно (p <0,001), в категории 2 – 48,5% против 0, в категории 3 – 65,5 против 23,1% (p=0,011), в категории 4 – 0.
Park J.-H. et al. [87] ретроспективно проанализировали результаты применения конверсионной хирургии в отношении 92 пациентов с РЖ IV стадии, 48 из которых хирургическое лечение было выполнено на первом этапе, а 44 – после ХТ (конверсионное лечение). Двугодичная выживаемость пациентов после хирургического и конверсионного лечения в категориях 1–4 составила соответственно 48,6 и 41,7% (p=0,829), 52,6 и 40,0% (p=0,855), 50,0 и 75,0% (p=0,027), 0% и 66,7% (p=0,083). Различий в выживаемости пациентов 1-й и 2-й категорий в зависимости от характера лечения выявлено не было. Двугодичная выживаемость больных 3-й и 4-й категорий была значимо выше при конверсионном лечении, чем при одном только хирургическом лечении (p=0,014). При наличии перитонеальной диссеминации 2-годичная выживаемость пациентов, получивших хирургическое или конверсионное лечение, значимо различалась, составив 41,7 и 72,7% (p=0,014) соответственно.
Представленные работы еще раз подтвердили ключевое значение операций в объеме R0 для улучшения отдаленных результатов лечения. Наибольший выигрыш от конверсионного лечения могут получить пациенты 1-й категории. При изолированном метастатическом поражении брюшины основное значение для увеличения выживаемости больных имеет ХТ. Дополнение эффективной ХТ хирургической операцией в объеме R0 после достижения резектабельности процесса способствует увеличению продолжительности жизни больных даже при множественных метастазах. Однако представленные исследования носят ретроспективный характер, когда уже известны и истинная распространенность болезни, и результаты лечения. Возможность применения классификации Yoshidа в качестве стандарта в отношении пациентов при первичном выявлении заболевания остается неясной, т.к. часто трудно определить истинную резектабельность опухолевого процесса на момент постановки диагноза и предсказать ответ на ХТ [40].
В связи с этим интересны сведения крупного международного ретроспективного анализа результатов конверсионного лечения, представленные Yoshida в 2022 г. [88], – исследование CONVO-GC- 1, в которое были включены 1206 больных РЖ IV стадии из Японии (n=776), Кореи (n=323) и Китая (n=107). Всем пациентам было проведено конверсионное лечение.
В категории 1–4 было включено соответственно 445, 344, 300 и 117 больных.
ХТ представляла собой современные платино-фторпиримидиновые дуплеты±доцетаксел. Объективный ответ достигнут в категории 1 у 54,1% пациентов, в категории 2 – у 72,6%, в категории 3 – у 39,0%, в категории 4 – у 61,6%. Медиана продолжительности предоперационной ХТ для всей группы составила 124 (81–209) дня, в категории 1 – 92 (75–148,5) дня, в категории 2 – 135,5 (84,25–240), в категории 3 – 158 (92–236), в категории 4 – 174 (117–272) дня, т.е. продолжительность ХТ увеличивалась с ростом распространенности опухолевого процесса.
МОВ всей когорты пациентов была необычно высокой: 36,7 месяца; в категории 1 – 38,4, в категории 2 – 46,6, в категории 3 – 33,4, в категории 4 – 34,1 месяца. Статистически значимых различий ОВ пациентов разных категорий не получено. Анализ выживаемости больных в зависимости от наличия резидуальной опухоли показал, что при выполнении операции в объеме R0 МОВ была значимо больше (p<0,001), чем при операциях в объеме R1, R2 – 56,6, 25,8 и 21,7 месяца соответственно. Аналогичная закономерность наблюдалась в каждой категории пациентов. После резекции в объеме R0 МОВ больных в категориях 1–3 составила соответственно 47,8, 116,7, и 44,8 месяца и не была достигнута пациентами 4-й категории, с высокой степенью достоверности превысив МОВ пациентов после резекций в объеме R1 и R2 (p<0,001). Хирургическое лечение в объеме R0 привело к значимому улучшению выживаемости и при макроскопически определяемых метастазах по брюшине, и при cyt+, и при поражении забрюшинных лимфоузлов. После конверсионного лечения больных РЖ с метастазами в печени МОВ пациентов 1-й и 2-й категорий составила соответственно 95,2 и 46,6 месяца, в 4-й категории медиана не достигнута (p=0,18). Эффективность лечения при операциях в объеме R0 не зависела от исходного количества метастазов. МОВ больных с 1–3 и более очагами составили соответственно 95,2, 46,6с и 56,6 месяца без статистически значимых различий (p=0,26). Анализ ОВ пациентов, оперированных в объеме R0, в зависимости от патоморфологического ответа первичного очага на ХТ показал, что при полном/частичном ответе МОВ составила 95,2, у больных без патоморфологического ответа – только 36,2 месяца. (p<0,0001). Клинический объективный ответ опухоли на ХТ в данных подгруппах также значимо различался: 66 и 53% (p=0,0021). Полученные данные свидетельствуют: лекарственный патоморфоз опухоли является прогностическим маркером, возможно, более чувствительным, чем клинический ответ на ХТ.
Таким образом, результаты крупного ретроспективного исследования Yoshida [88], представленные в 2022 г., отличаются от опубликованных ранее. Самое интересное, что не выявлено значимых различий выживаемости пациентов различных категорий и выживаемость пациентов категории 1 не превысила показателей остальных категорий. Авторы предположили, что это может быть связано с гетерогенностью подгрупп, обусловленной отсутствием единых критериев отбора пациентов для конверсионного лечения и различными показаниями к хирургическому лечению РЖ IV стадии в разных странах, что ведет к разнородности результатов, получаемых при ретроспективных анализах.
Разнородность результатов ретроспективных исследований свидетельствует об острой необходимости проведения проспективных рандомизированных исследований III фазы при РЖ IV стадии для определения показаний к конверсионному лечению и критериев отбора пациентов. Следует отметить, что в настоящее время в Европе проводится исследование RENAISSANCE (AIO-FLOT5), посвященное решению вышеуказанных проблем при олигометастатическом РЖ. Будем надеяться, что его результаты ответят на вопрос о целесообразности включения хирургического метода в план лечения больных неоперабельным РЖ.
Заключение
Больные диссеминированным РЖ представляют собой чрезвычайно гетерогенную популяцию по характеристикам как самих пациентов (возраст, пол, соматический статус, число и тяжесть сопутствующих заболеваний и т. д.), так и непосредственно опухолевого процесса (гистологическое строение, молекулярные биомаркеры, распространенность и характер течения заболевания и т.д.). Основой лечения РЖ IV стадии по-прежнему является противоопухолевая лекарственная терапия. Одно только хирургическое лечение не приводит к увеличению продолжительности жизни пациентов, однако выполнение условно-радикальных операции на фоне регрессии опухоли, вызванной эффективной ХТ, может существенно улучшать отдаленные результаты лечения. Такой мультимодальный подход находит все больше сторонников в крупных онкологических клиниках всего мира. Наиболее перспективной стратегией является конверсионное лечение, включающее проведение лекарственной терапии с последующим выполнением хирургического пособия в объеме R0 в случае достижения резектабельности опухолевых проявлений. Именно «радикализм» операции является залогом длительной выживаемости пациентов. Но такая тактика эффективна только при полном или частичном ответе опухоли на ХТ. Основные кандидаты на конверсионное лечение – это пациенты с олигометастатической болезнью, однако и при более обширной диссеминации также есть шанс на конверсию РЖ IV стадии в резектабельное состояние. Максимальный регресс опухолевых проявлений возможен только после интенсивной лекарственной терапии, обеспечивающей объективный эффект и стойкий контроль заболевания. Современная ХТ в комбинации с таргетными препаратами и/или ингибиторами контрольных точек может повышать частоту выполнения операций в объеме R0, однако результаты исследований в этом направлении неизвестны. Не определены ни оптимальные режимы, ни рациональная длительность предоперационной лекарственной терапии, не установлены сроки выполнения хирургического пособия, не выработана тактика послеоперационного ведения пациентов.
В отсутствие проспективных рандо-мизированных исследований не раз-работаны четкие показания и критерии отбора больных, в связи с чем показания к конверсионному лечению носят в основном индивидуальный характер.
Таким образом, несмотря на обнадеживающие результаты представленных в литературе ретроспективных работ и накопленный позитивный опыт, окончательные выводы о роли и месте хирургического метода в лечении больных РЖ IV стадии делать преждевременно, необходимо проведение проспективных многоцентровых рандомизированных клинических исследований.
Дополнительная информация
Публикация статьи осуществляется в рамках диссертационной работы на соискание ученой степени докт. мед. наук: «Оптимизация и персонализация тактики лечения больных диссеминированной аденокарциномой желудка и пищеводно-желудочного перехода (раком желудка) на основе клинических, патоморфологических и молекулярно-генетических характеристик».



